
|
Maryland Register
Issue Date: February 23, 2024
Volume 51 • Issue 4 • Pages 193 — 220
IN THIS ISSUE
Judiciary
Regulatory Review and Evaluation
Regulations
Special Documents
General Notices
|
Pursuant to State Government Article, §7-206, Annotated Code of Maryland, this issue contains all previously unpublished documents required to be published, and filed on or before February 5, 2024 5 p.m.
Pursuant to State Government Article, §7-206, Annotated Code of Maryland, I hereby certify that this issue contains all documents required to be codified as of February 5, 2024.
Gail S. Klakring
Acting Administrator, Division of State Documents
Office of the Secretary of State
|

|
Information About the Maryland Register and COMAR
MARYLAND REGISTER
The Maryland Register is an official State publication published every
other week throughout the year. A cumulative index is published quarterly.
The Maryland Register is the temporary supplement to the Code of
Maryland Regulations. Any change to the text of regulations published in COMAR, whether by adoption, amendment,
repeal, or emergency action, must first be published in the Register.
The following information is also published regularly in the Register:
• Governor’s Executive Orders
• Attorney General’s Opinions in full text
• Open Meetings Compliance Board Opinions in full text
• State Ethics Commission Opinions in full text
• Court Rules
• District Court Administrative Memoranda
• Courts of Appeal Hearing Calendars
• Agency Hearing and Meeting Notices
• Synopses of Bills Introduced and Enacted
by the General Assembly
• Other documents considered to be in the public interest
CITATION TO THE
MARYLAND REGISTER
The Maryland Register is cited by volume, issue, page number, and date.
Example:
• 19:8 Md. R. 815—817 (April 17,
1992) refers to Volume 19, Issue 8, pages 815—817 of the Maryland Register
issued on April 17, 1992.
CODE OF MARYLAND
REGULATIONS (COMAR)
COMAR is the official compilation of all regulations issued by agencies
of the State of Maryland. The Maryland Register is COMAR’s temporary
supplement, printing all changes to regulations as soon as they occur. At least
once annually, the changes to regulations printed in the Maryland Register are
incorporated into COMAR by means of permanent supplements.
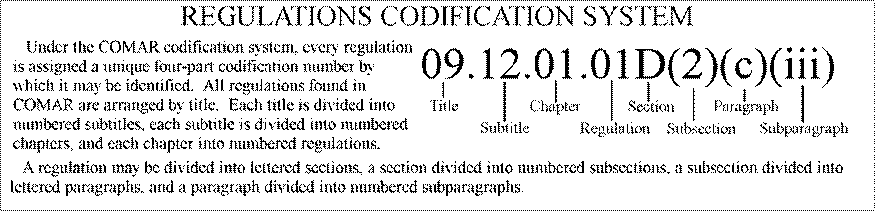
CITATION TO COMAR
REGULATIONS
COMAR regulations are cited by title number, subtitle number, chapter
number, and regulation number. Example: COMAR 10.08.01.03 refers to Title 10,
Subtitle 08, Chapter 01, Regulation 03.
DOCUMENTS INCORPORATED
BY REFERENCE
Incorporation by reference is a legal device by which a document is made
part of COMAR simply by referring to it. While the text of an incorporated
document does not appear in COMAR, the provisions of the incorporated document
are as fully enforceable as any other COMAR regulation. Each regulation that
proposes to incorporate a document is identified in the Maryland Register by an
Editor’s Note. The Cumulative Table of COMAR Regulations Adopted, Amended or
Repealed, found online, also identifies each regulation incorporating a
document. Documents incorporated by reference are available for inspection in
various depository libraries located throughout the State and at the Division
of State Documents. These depositories are listed in the first issue of the
Maryland Register published each year. For further information, call
410-974-2486.
HOW TO RESEARCH REGULATIONS
An
Administrative History at the end of every COMAR chapter gives information
about past changes to regulations. To determine if there have been any
subsequent changes, check the ‘‘Cumulative Table of COMAR Regulations Adopted,
Amended, or Repealed’’ which is found online at http://www.dsd.state.md.us/PDF/CumulativeTable.pdf.
This table lists the regulations in numerical order, by their COMAR number,
followed by the citation to the Maryland Register in which the change occurred.
The Maryland Register serves as a temporary supplement to COMAR, and the two
publications must always be used together. A Research Guide for Maryland
Regulations is available. For further information, call 410-260-3876.
SUBSCRIPTION
INFORMATION
For subscription forms for the Maryland Register and COMAR, see the back
pages of the Maryland Register. Single issues of the Maryland Register are $15.00
per issue.
CITIZEN PARTICIPATION IN
THE REGULATION-MAKING PROCESS
Maryland citizens and other interested
persons may participate in the process by which administrative regulations are
adopted, amended, or repealed, and may also initiate the process by which the
validity and applicability of regulations is determined. Listed below are some
of the ways in which citizens may participate (references are to State
Government Article (SG),
Annotated
Code of Maryland):
• By submitting data or views on proposed
regulations either orally or in writing, to the proposing agency (see
‘‘Opportunity for Public Comment’’ at the beginning of all regulations
appearing in the Proposed Action on Regulations section of the Maryland
Register). (See SG, §10-112)
• By petitioning an agency to adopt, amend,
or repeal regulations. The agency must respond to the petition. (See SG
§10-123)
• By petitioning an agency to issue a
declaratory ruling with respect to how any regulation, order, or statute
enforced by the agency applies. (SG, Title 10, Subtitle 3)
• By petitioning the circuit court for a
declaratory judgment
on
the validity of a regulation when it appears that the regulation interferes
with or impairs the legal rights or privileges of the petitioner. (SG, §10-125)
• By inspecting a certified copy of any
document filed with the Division of State Documents for publication in the
Maryland Register. (See SG, §7-213)
Maryland Register (ISSN
0360-2834). Postmaster: Send
address changes and other mail to: Maryland Register, State House, Annapolis,
Maryland21401. Tel. 410-260-3876. Published biweekly, with cumulative indexes
published quarterly, by the State of Maryland, Division of State Documents,
State House, Annapolis, Maryland 21401. The subscription rate for the Maryland
Register is $225 per year (first class mail). All subscriptions post-paid to
points in the U.S. periodicals postage paid at Annapolis, Maryland and
additional mailing offices.
Wes Moore, Governor; Susan C. Lee, Secretary of State; Gail S. Klakring, Administrator; Mary D. MacDonald, Senior Editor,
Maryland Register and COMAR; Elizabeth
Ramsey, Editor, COMAR Online, and Subscription Manager; Tami Cathell,
Help Desk, COMAR and Maryland Register Online.
Front cover: State House, Annapolis, MD, built 1772—79.
Illustrations by Carolyn Anderson, Dept. of General Services
Note: All
products purchased are for individual use only. Resale or other compensated
transfer of the information in printed or electronic form is a prohibited
commercial purpose (see State Government Article, §7-206.2, Annotated Code of
Maryland). By purchasing a product, the buyer agrees that the purchase is for
individual use only and will not sell or give the product to another individual
or entity.
Closing Dates for the Maryland
Register
Schedule of Closing Dates and
Issue Dates for the
Maryland Register ................................................................ 196
COMAR Research Aids
Table
of Pending Proposals ....................................................... 197
Index of COMAR Titles Affected in This
Issue
COMAR
Title Number and Name Page
08 Department of Natural Resources ................................... 202
09 Maryland Department of Labor ...................................... 202
10 Maryland Department of Health ..................................... 203
PERSONS
WITH DISABILITIES
Individuals
with disabilities who desire assistance in using the publications and services
of the Division of State Documents are encouraged to call (410) 974-2486, or
(800) 633-9657, or FAX to (410) 974-2546, or through Maryland Relay.
The Judiciary
SUPREME COURT OF MARYLAND
DISCIPLINARY PROCEEDINGS ................................ 200
Regulatory Review and Evaluation
DEPARTMENT OF TRANSPORTATION
Notice of Opportunity for Comment 201
Final Action on Regulations
08 DEPARTMENT OF NATURAL RESOURCES
BOATING—SPEED LIMITS AND OPERATION OF VESSELS
Ocean City—Back Bay Areas .............................................. 202
09 MARYLAND DEPARTMENT OF LABOR
STATE BOARD OF HEATING, VENTILATION, AIR-
CONDITIONING, AND REFRIGERATION
CONTRACTORS
General Regulations . 202
BOARD OF PLUMBING
Fees . 202
Proposed Action on Regulations
10 MARYLAND DEPARTMENT OF HEALTH
MEDICAL CARE PROGRAMS
General Medical Assistance Provider
Participation
Criteria . 203
Home and Community-Based Services
Waiver for Individuals
with Brain Injury ............................................................... 204
Targeted Case Management for People
with Developmental
Disabilities . 205
Early and Periodic Screening,
Diagnosis, and Treatment:
Nursing Services for Individuals
Younger than 21 Years
Old . 206
Home and Community-Based Services
Waiver for Children
with Autism Spectrum Disorder 207
Maryland Medicaid Managed Care
Program: Rare and
Expensive Case Management 209
1915(i) Intensive Behavioral Health
Services for Children,
Youth, and Families .......................................................... 210
DANGEROUS DEVICES AND SUBSTANCES
Controlled Dangerous Substances 211
Special Documents
DEPARTMENT OF THE ENVIRONMENT
SUSQUEHANNA RIVER BASIN COMMISSION
Commission Meeting . 219
WATER AND SCIENCE ADMINISTRATION
Water Quality Certification 23-WQC-0030 . 219
General Notices
STATE COLLECTION AGENCY LICENSING BOARD
Public Meeting . 220
MARYLAND DEPARTMENT OF HEALTH
Public Meeting .................................................................... 220
MARYLAND DEPARTMENT OF HEALTH/HARM
REDUCTION STANDING ADVISORY COMMITTEE
Public Meeting . 220
BOARD OF PUBLIC ACCOUNTANCY
Public Meeting . 220
Public Hearing . 220
STATE ADVISORY COUNCIL ON QUALITY CARE AT
THE END OF LIFE
Public Meeting .................................................................... 220
BOARD OF WATERWORKS AND WASTE SYSTEMS
OPERATORS
Public Meeting . 220
COMAR
Online
The Code of Maryland
Regulations is available at www.dsd.state.md.us as a free service of the Office
of the Secretary of State, Division of State Documents. The full text of
regulations is available and searchable. Note, however, that the printed COMAR
continues to be the only official and enforceable version of COMAR.
The Maryland Register is
also available at www.dsd.state.md.us.
For additional
information, visit www.dsd.maryland.gov, Division
of State Documents, or call us at (410) 974-2486 or 1 (800) 633-9657.
Availability
of Monthly List of
Maryland Documents
The Maryland Department of
Legislative Services receives copies of all publications issued by State
officers and agencies. The Department prepares and distributes, for a fee, a
list of these publications under the title ‘‘Maryland Documents’’. This list is
published monthly, and contains bibliographic information concerning regular
and special reports, bulletins, serials, periodicals, catalogues, and a variety
of other State publications. ‘‘Maryland Documents’’ also includes local
publications.
Anyone wishing to receive
‘‘Maryland Documents’’ should write to: Legislative Sales, Maryland Department
of Legislative Services, 90 State Circle, Annapolis, MD 21401.
CLOSING DATES AND ISSUE DATES THROUGH
DECEMBER 2024†
|
Issue
Date
|
Emergency
and Proposed
Regulations
5
p.m.*
|
Notices,
etc.
10:30
a.m.
|
Final
Regulations
10:30
a.m.
|
|
2024
|
|
March 8
|
February 16**
|
February 26
|
February 28
|
|
March 22
|
March 4
|
March 11
|
March 13
|
|
April 5
|
March 18
|
March 25
|
March 27
|
|
April 19
|
April 1
|
April 8
|
April 10
|
|
May 3
|
April 15
|
April 22
|
April 24
|
|
May 17
|
April 29
|
May 6
|
May 8
|
|
May 31
|
May 13
|
May 20
|
May 22
|
|
June 14
|
May 24**
|
June 3
|
June 5
|
|
June 28
|
June 10
|
June 17
|
June 18**
|
|
July 12
|
June 24
|
July 1
|
July 3
|
|
July 26
|
July 8
|
July 15
|
July 17
|
|
August 9
|
July 22
|
July 29
|
July 31
|
|
August 23
|
August 5
|
August 12
|
August 14
|
|
September 6
|
August 19
|
August 26
|
August 28
|
|
September 20
|
August 30**
|
September 9
|
September 11
|
|
October 4
|
September 16
|
September 23
|
September 25
|
|
October 18
|
September 30
|
October 7
|
October 9
|
|
November 1
|
October 11**
|
October 21
|
October 23
|
|
November 15
|
October 28
|
November 4
|
November 6
|
|
December
2***
|
November 8**
|
November 18
|
November 20
|
|
December 13
|
November 25
|
December 2
|
December 4
|
|
December 27
|
December 9
|
December 16
|
December 18
|
† Please
note that this table is provided for planning purposes and that the Division of
State Documents (DSD) cannot guarantee submissions will be published in an
agency’s desired issue. Although DSD strives to publish according to the
schedule above, there may be times when workload pressures prevent adherence to
it.
* Also note that proposal deadlines are for
submissions to DSD for publication
in the Maryland Register and do not take into account the 15-day AELR review
period. The due date for documents containing 8 to 18 pages is 48 hours before
the date listed; the due date for documents exceeding 18 pages is 1 week before
the date listed.
NOTE: ALL DOCUMENTS MUST BE SUBMITTED IN TIMES NEW
ROMAN, 9-POINT, SINGLE-SPACED FORMAT. THE PAGE COUNT REFLECTS THIS FORMATTING.
** Note closing date changes.
*** Note issue date changes.
The regular closing date for Proposals and
Emergencies is Monday.
Cumulative Table of COMAR Regulations
Adopted, Amended, or Repealed
This
table, previously printed in the Maryland Register lists the regulations, by
COMAR title, that have been adopted, amended, or repealed in the Maryland
Register since the regulations were originally published or last supplemented
in the Code of Maryland Regulations (COMAR). The table is no longer printed
here but may be found on the Division of State Documents website at
www.dsd.state.md.us.
Table of Pending Proposals
The table below lists proposed changes to
COMAR regulations. The proposed changes are listed by their COMAR number,
followed by a citation to that issue of the Maryland Register in which the
proposal appeared. Errata and corrections pertaining to proposed regulations
are listed, followed by “(err)” or “(corr),” respectively. Regulations
referencing a document incorporated by reference are followed by “(ibr)”. None
of the proposals listed in this table have been adopted. A list of adopted
proposals appears in the Cumulative Table of COMAR Regulations Adopted,
Amended, or Repealed.
05 DEPARTMENT OF HOUSING
AND COMMUNITY DEVELOPMENT
05.03.09.01—.11 •
50:7 Md. R. 304 (4-7-23)
05.20.05.01—.09 • 51:3 Md. R. 156 (2-9-24)
08 DEPARTMENT OF NATURAL
RESOURCES
08.02.03.01,.10,.12—.14
• 51:1 Md. R. 17 (1-12-24)
08.02.03.07 •
51:1 Md. R. 20 (1-12-24)
08.02.04.04 •
51:1 Md. R. 27 (1-12-24)
08.02.05.07 •
51:1 Md. R. 29 (1-12-24)
08.02.15.04,.05,.07
• 50:20 Md. R. 904 (10-6-23)
08.02.23.04 •
51:1 Md. R. 27 (1-12-24)
08.02.26.01—.06 •
51:1 Md. R. 30 (1-12-24)
09 MARYLAND DEPARTMENT OF LABOR
09.08.01.18 • 50:25 Md. R. 1093 (12-15-23)
09.08.07.02 • 50:25 Md. R. 1093 (12-15-23)
09.09.01.03 • 51:1 Md. R. 32 (1-12-24)
09.10.02.43,.53 • 50:24 Md. R. 1046 (12-1-23)
09.11.09.02 • 50:26 Md. R. 1135 (12-29-23)
09.13.05.03 • 50:26 Md. R. 1136 (12-29-23)
09.14.02.01,.02,.06-1,.06-2 • 50:25 Md. R. 1094
(12-15-23)
09.14.07.03,.05 • 50:25 Md. R. 1094 (12-15-23)
09.15.01.03 • 50:25 Md. R. 1094 (12-15-23)
09.16.01.08 • 50:25 Md. R. 1095 (12-15-23)
09.17.03.03 • 50:25 Md. R. 1096 (12-15-23)
09.18.01.03 • 50:25 Md. R. 1097 (12-15-23)
09.21.04.03 • 50:26 Md. R. 1136 (12-29-23)
09.22.01.12 • 50:25 Md. R. 1099 (12-15-23)
09.23.04.03 •
50:26 Md. R. 1137 (12-29-23)
09.24.01.09 •
50:26 Md. R. 1138 (12-29-23)
09.28.03.03 •
50:26 Md. R. 1139 (12-29-23)
09.33.02.01—.09 •
50:25 Md. R. 1100 (12-15-23)
09.34.01.01,.02,.04,.05
• 51:1 Md. R. 33 (1-12-24)
09.34.01.13 •
51:1 Md. R. 34 (1-12-24)
09.34.02.01 •
51:1 Md. R. 33 (1-12-24)
09.34.03.01,.02 •
51:1 Md. R. 35 (1-12-24)
09.34.04.01—.03 •
51:1 Md. R. 33 (1-12-24)
09.36.07.02 •
50:17 Md. R. 772 (8-25-23)
09.36.08.02 •
50:25 Md. R. 1101 (12-15-23)
10 MARYLAND DEPARTMENT OF HEALTH
Subtitle 09 (2nd volume)
10.09.02.05,.07 •
50:24 Md. R. 1048 (12-1-23) (ibr)
10.09.06.09 •
51:1 Md. R. 36 (1-12-24)
10.09.10.07,.08 •
51:2 Md. R. 78 (1-26-24)
10.09.11.11 •
51:2 Md. R. 79 (1-26-24)
10.09.16.01—.12 •
51:3 Md. R. 159 (2-9-24)
10.09.21.02—.06 •
51:2 Md. R. 82 (1-26-24)
10.09.24.02,.07,.12
• 51:2 Md. R. 79 (1-26-24)
10.09.24.03 •
50:18 Md. R. 814 (9-8-23)
10.09.33.09 •
51:3 Md. R. 161 (2-9-24)
10.09.36.01,.04 •
51:4 Md. R. 203 (2-23-24)
10.09.36.03-2 •
50:18 Md. R. 814 (9-8-23)
10.09.39.02,.06 •
50:24 Md. R. 1049 (12-1-23)
10.09.43.10,.13 • 51:2 Md. R. 79 (1-26-24)
10.09.44.01,.15,.21,.23 • 51:3 Md. R. 162 (2-9-24)
10.09.46.12 • 51:4 Md. R. 204 (2-23-24)
10.09.48.08 • 51:4 Md. R. 205 (2-23-24)
10.09.53.04,.05 • 51:4 Md. R. 206 (2-23-24)
10.09.56.02,.04,.10,.14—.17,.19,.21,.22 • 51:4 Md. R.
207 (2-23-24)
10.09.64.01—.09 • 51:3 Md. R. 164 (2-9-24)
10.09.69.02,.11,.12 • 51:4 Md. R. 209 (2-23-24)
10.09.80.01,.05,.08 • 51:1 Md. R. 37 (1-12-24)
10.09.89.14 • 51:4 Md. R. 210 (2-23-24)
10.09.92.04,.05 • 51:1 Md. R. 38 (1-12-24)
Subtitles 10—22 (3rd volume)
10.11.08.01—.06 •
51:1 Md. R. 39 (1-12-24)
10.15.06.02,.03,.05,.10,.11 • 51:2 Md. R. 82 (1-26-24) (ibr)
10.18.05.01—.03 •
51:3 Md. R. 166 (2-9-24)
10.18.06.05,.08,.10
• 51:3 Md. R. 166 (2-9-24)
10.19.03.01—.20 •
51:4 Md. R. 211 (2-23-24)
10.21.31.01—.06 •
51:3 Md. R. 167 (2-9-24)
Subtitles 23—36 (4th volume)
10.24.10.01 • 50:24
Md. R. 1050 (12-1-23) (ibr)
10.25.07.02,.04,.05,.09
• 51:1 Md. R. 41 (1-12-24)
10.25.18.01—.04,.06,.07,.09—.11
• 51:1 Md. R. 43 (1-12-24)
10.27.01.05 •
50:20 Md. R. 907 (10-6-23)
10.32.01.10 •
51:2 Md. R. 83 (1-26-24)
10.34.42.01—.03 •
51:2 Md. R. 84 (1-26-24)
Subtitles 37—52 (5th volume)
10.41.01.01—.04 •
50:16 Md. R. 738 (8-11-23)
10.41.02.01,.02,.04
• 50:16 Md. R. 738 (8-11-23)
10.41.03.02,.03,.05,.06
• 50:16 Md. R. 738 (8-11-23)
10.41.04.01,.02,.06,.08
• 50:16 Md. R. 738 (8-11-23)
10.41.05.01—.07 •
50:16 Md. R. 738 (8-11-23)
10.41.08.01-1,.02,.06,.08,.11,.12,.14
• 50:16 Md. R. 738 (8-11-23)
10.41.09.02 •
50:16 Md. R. 738 (8-11-23)
10.41.11.01—.10 •
50:16 Md. R. 738 (8-11-23)
10.41.13.02,.04 •
50:16 Md. R. 738 (8-11-23)
10.44.01.01—.39 •
50:20 Md. R. 911 (10-6-23)
10.44.19.05—.12 •
50:24 Md. R. 1051 (12-1-23)
10.44.20.02 •
50:20 Md. R. 918 (10-6-23)
10.44.22.02,.04—.06,.08—.15
• 50:20 Md. R. 918 (10-6-23)
10.46.09.01—.04 •
51:2 Md. R. 85 (1-26-24)
Subtitles 53—68 (6th volume)
10.53.08.05 •
50:17 Md. R. 773 (8-25-23)
10.53.09.01—.04 •
50:17 Md. R. 773 (8-25-23)
10.60.01.03,.05 •
50:18 Md. R. 816 (9-8-23)
10.60.02.08,.09 •
50:25 Md. R. 1102 (12-15-23)
10.60.03.01—.05 •
50:25 Md. R. 1102 (12-15-23)
10.63.02.02 •
51:3 Md. R. 168 (2-9-24)
10.63.03.20,.21 •
51:3 Md. R. 168 (2-9-24)
10.63.07.02,.03,.05,.11
• 51:3 Md. R. 173 (2-9-24)
10.65.02.06,.09 •
51:2 Md. R. 86 (1-26-24)
10.65.03.02—.09 •
51:2 Md. R. 86 (1-26-24)
10.65.04.01—.06 •
51:2 Md. R. 86 (1-26-24)
10.65.05.01—.04 •
51:2 Md. R. 86 (1-26-24)
10.65.06.01,.02 •
51:2 Md. R. 86 (1-26-24)
10.65.09.01—.06 •
51:2 Md. R. 86 (1-26-24)
10.67.01.01 •
51:3 Md. R. 164 (2-9-24)
10.67.02.01 •
51:3 Md. R. 174 (2-9-24)
10.67.04.03-1,.03-2,.15,.19,.19-4
• 51:3 Md. R. 174 (2-9-24)
10.67.04.20 •
50:24 Md. R. 1049 (12-1-23)
10.67.05.02 •
51:3 Md. R. 174 (2-9-24)
10.67.06.26 •
51:3 Md. R. 164 (2-9-24)
10.67.06.26-1,.26-3,.27,.30
• 51:3 Md. R. 174 (2-9-24)
10.67.06.28 •
50:24 Md. R. 1049 (12-1-23)
10.67.08.03 • 51:3 Md. R. 174 (2-9-24)
11 DEPARTMENT OF
TRANSPORTATION
Subtitles 11—23 (MVA)
11.11.13.04 •
51:3 Md. R. 177 (2-9-24)
11.12.01.14 •
50:15 Md. R. 698 (7-28-23)
11.13.10.04,.10,.14,.20 • 51:3 Md. R. 177 (2-9-24)
11.20.01.01,.03—.12,.14—.33 • 51:3 Md. R. 178
(2-9-24)
12 DEPARTMENT OF PUBLIC
SAFETY AND CORRECTIONAL SERVICES
12.11.10.06 •
51:2 Md. R. 95 (1-26-24)
13A STATE BOARD OF EDUCATION
13A.07.06.01—.15
• 50:14 Md. R. 621 (7-14-23) (ibr)
13A.08.01.17 •
50:20 Md. R. 924 (10-6-23)
13A.12.01.01—.14
• 50:14 Md. R. 633 (7-14-23)
13A.12.02.01—.29
• 50:14 Md. R. 633 (7-14-23)
13A.12.03.01—.12
• 50:14 Md. R. 633 (7-14-23)
13A.12.04.01—.16
• 50:14 Md. R. 633 (7-14-23)
13A.12.05.01—.15
• 50:14 Md. R. 633 (7-14-23)
13A.12.06.01—.09
• 50:14 Md. R. 633 (7-14-23)
13A.12.07.01—.08
• 50:14 Md. R. 633 (7-14-23)
50:15 Md. R. 707 (7-28-23) (err)
13A.15.05.06 •
51:1 Md. R. 50 (1-12-24)
13A.15.09.01 •
51:1 Md. R. 50 (1-12-24)
13A.15.10.06 •
51:1 Md. R. 50 (1-12-24)
13A.16.08.03 •
51:2 Md. R. 95 (1-26-24)
13A.16.09.01,.04
• 51:1 Md. R. 50 (1-12-24)
13A.16.10.02 •
51:2 Md. R. 95 (1-26-24)
13A.16.10.05 •
51:1 Md. R. 50 (1-12-24)
13A.17.10.02 •
51:2 Md. R. 95 (1-26-24)
13A.18.09.01,.04
• 51:1 Md. R. 50 (1-12-24)
13A.18.10.05 •
51:1 Md. R. 50 (1-12-24)
13B MARYLAND HIGHER
EDUCATION COMMISSION
13B.08.20.02—.13
• 50:4 Md. R. 158 (2-24-23)
14 INDEPENDENT AGENCIES
14.26.02.01—.12 •
50:26 Md. R. 1140 (12-29-23)
14.26.03.01—.13 •
50:26 Md. R. 1142 (12-29-23)
14.26.04.01—.13 •
51:3 Md. R. 183 (2-9-24)
14.26.06.01—.11 •
51:3 Md. R. 183 (2-9-24)
14.30.01.01 •
51:2 Md. R. 97 (1-26-24)
14.30.02.01—.05 •
51:2 Md. R. 97 (1-26-24)
14.30.03.01,.02 •
51:2 Md. R. 97 (1-26-24)
14.30.04.01—.12 •
51:2 Md. R. 97 (1-26-24)
14.30.05.01—.17 •
51:2 Md. R. 97 (1-26-24)
14.30.06.01,.02 •
51:2 Md. R. 97 (1-26-24)
14.30.07.01—.04 •
51:2 Md. R. 97 (1-26-24)
14.30.08.01—.26 •
51:2 Md. R. 97 (1-26-24)
14.30.09.01—.03 •
51:2 Md. R. 97 (1-26-24)
14.30.10.01—.24 •
51:2 Md. R. 97 (1-26-24)
14.30.11.01—.27 •
51:2 Md. R. 97 (1-26-24)
14.30.12.01—.05 •
51:2 Md. R. 97 (1-26-24)
14.30.13.01 •
51:2 Md. R. 97 (1-26-24)
14.30.14.01—.05 •
51:2 Md. R. 97 (1-26-24)
14.30.15.01,.02 •
51:2 Md. R. 97 (1-26-24)
14.32.01.01—.06 •
51:2 Md. R. 109 (1-26-24)
14.32.02.01—.22 •
51:2 Md. R. 109 (1-26-24)
14.32.03.01—.07 •
51:2 Md. R. 109 (1-26-24)
14.32.04.01—.06 •
51:2 Md. R. 109 (1-26-24)
14.32.05.01—.05 •
51:2 Md. R. 109 (1-26-24)
14.32.06.01—.03 •
51:2 Md. R. 109 (1-26-24)
14.32.07.01 •
51:2 Md. R. 109 (1-26-24)
14.32.08.01 •
51:2 Md. R. 109 (1-26-24)
14.34.01.01—.03 •
51:2 Md. R. 110 (1-26-24)
14.34.02.01 •
51:2 Md. R. 110 (1-26-24)
14.34.03.01 •
51:2 Md. R. 110 (1-26-24)
14.34.04.01—.17 •
51:2 Md. R. 110 (1-26-24)
14.34.05.01—.12 •
51:2 Md. R. 110 (1-26-24)
14.34.06.01—.04 •
51:2 Md. R. 110 (1-26-24)
14.38.01.03 •
50:23 Md. R. 1011 (11-17-23)
15 MARYLAND DEPARTMENT OF
AGRICULTURE
15.01.05.10 •
51:2 Md. R. 110 (1-26-24)
15.14.09.03 •
50:25 Md. R. 1103 (12-15-23)
15.14.12.02 •
51:2 Md. R. 111 (1-26-24)
18 DEPARTMENT OF
ASSESSMENTS AND TAXATION
18.01.02.03 •
51:3 Md. R. 184 (2-9-24)
18.06.03.01 •
51:3 Md. R. 184 (2-9-24)
21 STATE PROCUREMENT
REGULATIONS
21.03.05.03 •
51:2 Md. R. 112 (1-26-24)
21.05.07.01,.04,.05
• 51:2 Md. R. 112 (1-26-24)
21.05.08.05 •
51:2 Md. R. 112 (1-26-24)
21.05.09.05 •
51:2 Md. R. 112 (1-26-24)
21.11.01.06 •
51:2 Md. R. 112 (1-26-24)
21.11.14.02—.04,.06,.07,.09,.12
• 51:2 Md. R. 115 (1-26-24)
21.11.15.04 •
51:2 Md. R. 112 (1-26-24)
21.13.01.03,.15 •
51:2 Md. R. 112 (1-26-24)
26 DEPARTMENT OF THE
ENVIRONMENT
Subtitles 08—12 (Part 2)
26.11.40.02,.03 •
50:24 Md. R. 1059 (12-1-23)
Subtitles 19—28 (Part 4)
26.28.01.01—.03 •
50:25 Md. R. 1104 (12-15-23) (ibr)
26.28.02.01—.05 •
50:25 Md. R. 1104 (12-15-23)
26.28.03.01,.02 •
50:25 Md. R. 1104 (12-15-23)
26.28.04.01—.03 •
50:25 Md. R. 1104 (12-15-23)
26.30.01.01—.08 • 51:1 Md. R. 52 (1-12-24)
26.30.02.01—.09 • 51:1 Md. R. 52 (1-12-24)
26.30.03.01—.03 • 51:1 Md. R. 52 (1-12-24)
26.30.04.01,.02 • 51:1 Md. R. 52 (1-12-24)
30 MARYLAND INSTITUTE FOR
EMERGENCY MEDICAL SERVICES SYSTEMS (MIEMSS)
30.01.01.02 •
50:24 Md. R. 1061 (12-1-23)
30.01.02.01 •
50:24 Md. R. 1064 (12-1-23) (ibr)
30.02.02.04,.06—.09
• 50:24 Md. R. 1061 (12-1-23)
30.09.01.02 •
51:2 Md. R. 117 (1-26-24)
30.09.14.04 •
51:2 Md. R. 117 (1-26-24)
31 MARYLAND INSURANCE
ADMINISTRATION
31.10.30.03—.05 •
51:3 Md. R. 185 (2-9-24)
33 STATE BOARD OF
ELECTIONS
33.07.07.01 •
50:26 Md. R. 1147 (12-29-23)
33.07.09.01—.04 •
50:26 Md. R. 1147 (12-29-23)
33.11.01.04 •
50:26 Md. R. 1148 (12-29-23)
33.11.03.02,.08 •
50:26 Md. R. 1147 (12-29-23)
33.11.03.06 •
50:23 Md. R. 1029 (11-17-23)
33.11.04.03 •
50:23 Md. R. 1029 (11-17-23)
33.11.05.04 •
50:26 Md. R. 1148 (12-29-23)
33.16.03.01 •
50:26 Md. R. 1147 (12-29-23)
33.16.06.04 •
50:23 Md. R. 1029 (11-17-23)
33.17.06.10 • 50:26 Md. R. 1147
(12-29-23)
35 DEPARTMENT OF VETERANS
AFFAIRS
35.01.01.02 • 50:25 Md. R. 1115
(12-15-23)
35.03.01.03,.05,.09,.10 • 50:25
Md. R. 1115 (12-15-23)
51:1 Md. R. 58 (1-12-24) (err)
36 MARYLAND STATE LOTTERY
AND GAMING CONTROL AGENCY
36.03.01.02 • 50:26 Md. R. 1149
(12-29-23)
36.03.02.06,.12—.14,.16,.17 •
50:26 Md. R. 1149 (12-29-23)
36.03.03.01,.05—.07,.10 • 50:26
Md. R. 1149 (12-29-23)
36.03.06.03 • 50:26 Md. R. 1149
(12-29-23)
36.03.08.02,.04 • 50:26 Md. R.
1149 (12-29-23)
36.03.10.16,.20,.21,.34 • 50:26
Md. R. 1149 (12-29-23)
36.03.11.05 • 50:26 Md. R. 1149
(12-29-23)
36.04.01.11 • 50:26 Md. R. 1149
(12-29-23)
36.04.02.01,.02 • 50:26 Md. R.
1149 (12-29-23)
36.07.02.12,.18 • 50:26 Md. R.
1149 (12-29-23)
36.10.01.02 • 50:26 Md. R. 1149
(12-29-23)
36.10.02.10,.14 • 50:26 Md. R.
1149 (12-29-23)
36.10.03.02,.04 • 50:26 Md. R.
1149 (12-29-23)
36.10.04.02—.06 • 50:26 Md. R.
1149 (12-29-23)
36.10.05.01,.02 • 50:26 Md. R.
1149 (12-29-23)
36.10.06.02—.07,.09,.11 • 50:26
Md. R. 1149 (12-29-23)
36.10.10.03 • 50:26 Md. R. 1149
(12-29-23)
36.10.13.20,34,.40,.41 • 50:26
Md. R. 1149 (12-29-23)
36.10.14.03,.06 • 50:26 Md. R.
1149 (12-29-23)
36.10.15.03,.04 • 50:26 Md. R.
1149 (12-29-23)
36.11.02.20 • 50:26 Md. R. 1149
(12-29-23)
The Judiciary
SUPREME COURT OF MARYLAND
DISCIPLINARY PROCEEDINGS
This is to certify
that by an Order of this Court dated January 31, 2024, GAIL D. SAUSSER (CPF#
9912160125), as of January 31, 2024, Gail D. Sausser has resigned, effective
immediately and her name has been stricken from the register of attorneys in
this Court. Notice of this action is given in accordance with Maryland Rule
19-735(e).
* *
* * *
* * *
* *
This is to certify
that by an Order of this Court dated January 31, 2024, SCOTT GROVE PATTERSON
(CPF# 7201010153), as of January 31, 2024, Scott Grove Patterson has resigned,
effective immediately and his name has been stricken from the register of
attorneys in this Court. Notice of this action is given in accordance with
Maryland Rule 19-735(e).
[24-04-10]
Regulatory Review and Evaluation
Regulations
promulgated under the Administrative Procedure Act will undergo a review by the
promulgating agency in accordance with the Regulatory Review and Evaluation Act
(State Government Article, §§10-130 — 10-139; COMAR 01.01.2003.20). This
review will be documented in an evaluation report which will be submitted to
the General Assembly’s Joint Committee on Administrative, Executive, and
Legislative Review. The evaluation
reports have been spread over an 8-year period (see COMAR 01.01.2003.20 for the schedule). Notice that an evaluation report is available
for public inspection and comment will be published in this section of the
Maryland Register.
Title 10
MARYLAND DEPARTMENT OF HEALTH
Subtitle 09 MEDICAL CARE PROGRAMS
10.09.36 General Medical
Assistance Provider Participation Criteria
Authority: Health-General Article, §§2-104(b), 15-103, and 15-105,
Annotated Code of Maryland
Notice of Proposed Action
[23-343-P]
The Secretary of Health proposes to amend Regulations .01
and .04 under COMAR 10.09.36 General Medical Assistance Provider
Participation Criteria.
Statement of Purpose
The purpose of this action is to update existing regulations to
better align them with globally applicable payment procedures for participation
in the Maryland Medicaid Program.
Estimate of Economic Impact
The proposed action has no economic impact.
Economic Impact on Small Businesses
The proposed action has minimal or no economic impact on small
businesses.
Impact on Individuals with Disabilities
The proposed action has no impact on individuals with
disabilities.
Opportunity for Public Comment
Comments may be sent to Jourdan Green, Director, Office of
Regulation and Policy Coordination, Maryland Department of Health, 201 West
Preston Street, Room 512, Baltimore, MD 21201, or call 410-767-6499 (TTY
800-735-2258), or email to mdh.regs@maryland.gov. Comments will be accepted
through March 25, 2024. A public hearing has not been scheduled.
.01 Definitions.
A. (text unchanged)
B. Terms Defined.
(1)—(5) (text unchanged)
(6) “Current Procedural Terminology (CPT)” means the American
Medical Association’s uniform nomenclature for coding medical procedures and
services.
[(6)] (7)—[(8)] (9) (text
unchanged)
(10) “Healthcare Common Procedure Coding System (HCPCS)” means
the specified code set for procedures and services, according to the Health
Insurance Portability and Accountability Act (HIPAA).
[(9)] (11)—[(20)] (22)
(text unchanged)
.04 Payment Procedures.
A. The provider shall submit the request for payment of services
rendered according to procedures established by the Department and in the [form]
format designated by the Department.
B. The Department reserves the right to return to the provider,
before payment, all [invoices] claims not properly signed,
completed, and accompanied by properly completed forms required by the
Department.
[C. Payment advances are not made routinely.]
[D.] C. The Program will make no direct
payment to [recipients] participants.
D. All payments are contingent upon a provider’s full compliance
with the requirements of its enrollment and applicable conditions of
participation.
E. Unless the service is free to the general public, the
provider shall charge the Program its customary charge to the general public
for similar services.
F. Unless otherwise excepted, the provider shall be paid the
lesser of:
(1) The provider’s customary charge unless the service is free
to individuals not covered by the Program; or
(2) The Department’s applicable rate.
G. Unless otherwise excepted, if a service is free to
individuals not covered by the Program:
(1) The provider:
(a) May charge the Program; and
(b) Shall be reimbursed in accordance with §F of this
regulation; and
(2) The provider's reimbursement is not limited to the
provider's customary charge.
H. Providers may not bill the Department or the Program for:
(1) Completion of forms and reports;
(2) Broken or missed appointments;
(3) Professional services rendered by:
(a) Mail;
(b) Email; or
(c) Fax; or
(4) Providing a copy of a participant’s medical record when
requested by another licensed provider on behalf of the participant.
I. Unless otherwise
excepted, payments on Medicare claims are authorized, if:
(1) Services are covered by the Medicare Program;
(2) The provider accepts Medicare assignments;
(3) Medicare makes direct payment to the provider;
(4) Medicare has determined that services were medically
justified; and
(5) Initial billing is made directly to Medicare according to
Medicare guidelines.
J. Unless otherwise provided by regulation, supplemental
payments on Medicare claims are made subject to the following provisions:
(1) Deductible insurance will be paid in full;
(2) Beginning with August 1, 2010 dates of service and subject
to the limitations of the State budget, coinsurance shall be paid:
(a) In full for the following:
(i) Mental health services;
(ii) CPT codes that are priced by report;
(iii) Claims for anesthesia services;
(iv) Claims from a federally qualified health center; and
(v) HCPCS codes beginning with A through W; and
(b) For all other claims, at the lesser of:
(i) 100 percent of the coinsurance amount; or
(ii) The balance remaining after the Medicare payment is
subtracted from the Medicaid rate; and
(3) Services not covered by Medicare are payable according to §F
of this regulation.
K. An individual or entity who is employed by or under contract
to any group provider, clinic, or hospital may not bill for any service for
which reimbursement is sought by the group provider, clinic, or hospital.
LAURA HERRERA
SCOTT
Secretary of Health
Subtitle 09 MEDICAL
CARE PROGRAMS
10.09.46 Home and
Community-Based Services Waiver for Individuals with Brain Injury
Authority: Health-General Article, §§2-104(b), 15-103, and 15-105,
Annotated Code of Maryland
Notice of Proposed Action
[23-345-P]
The Secretary of Health proposes to amend Regulation .12
under COMAR 10.09.46 Home and Community-Based Services Waiver for
Individuals with Brain Injury.
Statement of Purpose
The purpose of this action is to
update the Brain Injury Waiver services fee schedule.
Estimate of Economic Impact
I. Summary of Economic Impact. Brain Injury Waiver: For
dates of service beginning July 1, 2023, the Maryland Medical Assistance
reimbursement rates for Brain Injury Waiver services will increase by 3
percent. This represents an estimated $446,521 increase in total expenditures.
II. Types of Economic Impact.
|
Impacted Entity
|
Revenue
(R+/R-)
Expenditure
(E+/E-)
|
Magnitude
|
|
A. On issuing agency:
|
|
|
|
Maryland Department of Health
|
(E+)
|
$446,521
|
|
B. On other State agencies:
|
NONE
|
|
|
C. On local governments:
|
NONE
|
|
|
|
|
|
|
|
Benefit
(+)
Cost
(-)
|
Magnitude
|
|
D. On regulated industries or
trade groups:
|
|
|
|
Maryland Medicaid
providers
|
(+)
|
$446,521
|
|
E. On other industries or
trade groups:
|
NONE
|
|
|
F. Direct and indirect
effects on public:
|
NONE
|
|
III. Assumptions. (Identified by Impact Letter and Number
from Section II.)
A. This amount assumes effective July 1, 2023, Brain Injury
providers will receive a 3 percent rate increase.
D. See A above.
Economic Impact on Small Businesses
The proposed action has a meaningful economic impact on small
businesses. An analysis of this economic impact follows:
Many of the providers of home and community-based services under
this chapter are small businesses that will benefit from additional rate
increases under the provisions of the proposed action. To the extent that Brain
Injury Waiver providers qualify as small businesses, they will benefit from the
rate increase included in this action.
Impact on Individuals with Disabilities
The proposed action has an impact on individuals with disabilities
as follows:
Individuals with disabilities receive services provided under this
chapter and will benefit to the extent that improved funding will enable
providers to maintain quality services.
Opportunity for Public Comment
Comments may be sent to Jourdan Green, Director, Office of
Regulation and Policy Coordination, Maryland Department of Health, 201 West
Preston Street, Room 512, Baltimore, MD 21201, or call 410-767-6499 (TTY
800-735-2258), or email to mdh.regs@maryland.gov. Comments will be accepted
through March 25, 2024. A public hearing has not been scheduled.
.12 Payment Procedures.
A.—B. (text unchanged)
C. Payments.
(1) (text unchanged)
(2) The Program shall pay according to the following
fee-for-service schedule:
(a) Residential habilitation reimbursed at:
(i) [$276.59] $266.64
from [July 1,] October
1, 2022 [to] through [September 30, 2022,] June
30, 2023, then effective [October
1, 2022] July 1, 2023 at [$266.64] $274.64 per day for
Level 1;
(ii) [$366.22] $353.06
from [July 1,] October 1, 2022 [to]
through [September 30, 2022,] June 30,2023, then effective [October 1, 2022] July 1, 2023 at [$353.06] $363.65 per day for
Level 2; and
(iii) [$506.64] $488.43
from [July 1,] October 1, 2022 [to]
through [September 30, 2022,] June 30, 2023, then effective [October 1, 2022] July 1, 2023 at [$488.43] $503.08 per day for
Level 3;
(b) Day habilitation reimbursed at:
(i) [$71.41] $68.84
from [July 1,] October 1, 2022 [to]
through [September 30, 2022,] June 30, 2023, then effective [October 1, 2022] July 1, 2023 at [$68.84] $70.91 per day for
Level 1;
(ii) [$124.57] $120.09
from [July 1,] October 1, 2022 [to]
through [September 30, 2022,] June 30, 2023, then effective [October 1, 2022] July 1, 2023 at [$120.09] $123.69 per day for
Level 2; and
(iii) [$175.24] $168.84
from [July 1,] October 1, 2022 [to]
through [September 30, 2022,] June 30, 2023, then effective [October 1, 2022] July 1, 2023 at [$168.84] $174.01 per day for
Level 3;
(c) Supported employment reimbursed at:
(i) [$42.36] $40.84
from [July 1,] October 1, 2022 [to]
through [September 30, 2022,] June 30, 2023, then effective [October 1, 2022] July 1, 2023 at [$40.84] $42.07 per day for
Level 1;
(ii) [$71.41] $68.84
from [July 1,] October 1, 2022 [to]
through [September 30, 2022,] June 30, 2023, then effective [October 1, 2022] July 1, 2023 at [$68.84] $70.91 per day for
Level 2; and
(iii) [$175.24] $168.84
from [July 1,] October 1, 2022 [to]
through [September 30, 2022,] June 30, 2023, then effective [October 1, 2022] July 1, 2023 at [$168.84] $174.01 per day for
Level 3; and
(d) Individual support services (ISS): reimbursed at the maximum
rate of [$8.6553] $8.3440 per 15 minutes
from [July 1,] October 1, 2022 [to]
through [September 30, 2022,] June 30, 2023, then effective [October 1, 2022] July 1, 2023 at [$8.3440] $8.6000 per 15 minutes.
(3)—(4) (text unchanged)
LAURA HERRERA
SCOTT
Secretary of Health
Subtitle 09 MEDICAL
CARE PROGRAMS
10.09.48 Targeted Case
Management for People with Developmental Disabilities
Authority: Health-General Article, §§2-104(b), 15-103, and 15-105,
Annotated Code of Maryland
Notice of Proposed Action
[23-340-P]
The Secretary of Health proposes to amend Regulation .08
under COMAR 10.09.48 Targeted Case Management for People with Developmental
Disabilities.
Statement of Purpose
The purpose of this action is to increase the reimbursement rate
for Developmental Disabilities Administration (DDA) targeted case management
(TCM) providers by 4 percent for Fiscal Year (FY) 2024.
Estimate of Economic Impact
I. Summary of Economic Impact. The proposed action
implements a 4 percent increase in the reimbursement rate for Developmental
Disabilities Administration (DDA) targeted case management (TCM) providers for
Fiscal Year 2024. The total impact of this change in rates for Fiscal Year 2024
is $3,486,940.
II. Types of Economic Impact.
|
Impacted Entity
|
Revenue
(R+/R-)
Expenditure
(E+/E-)
|
Magnitude
|
|
A. On issuing agency:
|
|
|
|
Maryland Department of Health
|
(E+)
|
$3,486,940
|
|
B. On other State agencies:
|
NONE
|
|
|
C. On local governments:
|
NONE
|
|
|
|
|
|
|
|
Benefit
(+)
Cost
(-)
|
Magnitude
|
|
D. On regulated industries or
trade groups:
|
|
|
|
Medicaid DDA TCM providers
|
(+)
|
$3,486,940
|
|
E. On other industries or
trade groups:
|
NONE
|
|
|
F. Direct and indirect
effects on public:
|
NONE
|
|
III. Assumptions. (Identified by Impact Letter and Number
from Section II.)
A. This amount assumes:
(1) Utilization of services covered under this chapter in Fiscal
Year 2024 will remain consistent with Fiscal Year 2023 rates.
(2) Effective July 1, 2023, Developmental Disabilities
Administration (DDA) targeted case management (TCM) providers will receive an
increased reimbursement rate of 4 percent.
(3) In total, these rate increases reflect an estimated $3,486,940
total fund increase. The 4 percent increase is subject to 48 percent federal
match ($1,813,209 general funds and $1,673,731 federal funds).
D. See A above.
Economic Impact on Small Businesses
The proposed action has a meaningful economic impact on small
businesses. An analysis of this economic impact follows:
To the extent that Medicaid enrolled targeted case management
providers qualify as small businesses, they will share in the benefit of this
rate increase.
Impact on Individuals with Disabilities
The proposed action has an impact on individuals with disabilities
as follows:
Individuals with disabilities receive services provided under this
chapter and will benefit to the extent that improved funding will enable
providers to maintain quality services.
Opportunity for Public Comment
Comments may be sent to Jourdan Green, Director, Office of
Regulation and Policy Coordination, Maryland Department of Health, 201 West
Preston Street, Room 512, Baltimore, MD 21201, or call 410-767-6499 (TTY
800-735-2258), or email to mdh.regs@maryland.gov. Comments will be accepted
through March 25, 2024. A public hearing has not been scheduled.
.08 Payment Procedures.
A. (text unchanged)
B. Payment Rates.
(1)—(5) (text unchanged)
(6) For all other services rendered to Maryland Medicaid
participants residing in counties other than those listed in §B(7) of this
regulation, providers shall be reimbursed:
[(a) For dates of service January 1, 2021 through October
31, 2021, $21.55 per unit of service;
(b) For dates of service November 1, 2021 through June 30, 2022,
$22.74 per unit of service;]
[(c)] (a) (text unchanged)
[(d)] (b) For dates of service October 1, 2022
through December 31, 2022, $27.02 per unit; [and]
[(e)] (c) For dates of service January 1, 2023
through June 30, 2023, $24.56 per unit[.]; and
(d) For dates of service July 1, 2023 through June 30, 2024,
$25.54 per unit.
(7) Providers rendering services to Maryland Medicaid participants
residing in Calvert, Charles, Frederick, Montgomery, and Prince George’s
counties shall be reimbursed:
[(a) For dates of service January 1, 2021 through October
31, 2021, $22.69 per unit;
(b) For dates of service November 1, 2021 through June 30, 2022,
$23.94 per unit;]
[(c)] (a) (text unchanged)
[(d)] (b) For dates of service October 1, 2022
through December 31, 2022, $28.45 per unit; [and]
[(e)] (c) For dates of service January 1, 2023
through June 30, 2023, $25.86 per unit[.]; and
(d) For dates of service July 1, 2023 through June 30, 2024,
$26.89 per unit.
C.—E. (text unchanged)
LAURA HERRERA SCOTT
Secretary of Health
Subtitle 09 MEDICAL
CARE PROGRAMS
10.09.53 Early and Periodic
Screening, Diagnosis, and Treatment: Nursing Services for Individuals Younger
than 21 Years Old
Authority: Health-General Article, §§2-104(b), 15-103, and 15-105,
Annotated Code of Maryland
Notice of Proposed Action
[23-347-P]
The Secretary of Health proposes to amend Regulations .04
and .05 under COMAR 10.09.53 Early and Periodic Screening, Diagnosis,
and Treatment: Nursing Services for Individuals Younger than 21 Years Old.
Statement of Purpose
The purpose of this action is to clarify covered private duty
nursing (PDN) services when provided to participants who are younger than 21
years old and eligible for early and periodic screening, diagnosis, and
treatment (EPSDT) services.
Estimate of Economic Impact
The proposed action has no economic impact.
Economic Impact on Small Businesses
The proposed action has minimal or no economic impact on small
businesses.
Impact on Individuals with Disabilities
The proposed action has an impact on individuals with disabilities
as follows:
Individuals with disabilities receive services provided under this
chapter and will benefit to the extent that clarification of the application of
the current regulations improve transparency to the medically complex
population served.
Opportunity for Public Comment
Comments may be sent to Jourdan Green, Director, Office of
Regulation and Policy Coordination, Maryland Department of Health, 201 West
Preston Street, Room 512, Baltimore, MD 21201, or call 410-767-6499 (TTY
800-735-2258), or email to mdh.regs@maryland.gov. Comments will be accepted
through March 25, 2024. A public hearing has not been scheduled.
.04 Covered Services.
A. The Program shall cover services rendered by a nurse, CNA, or
HHA when the:
(1)—(6) (text unchanged)
[(7) Services are determined medically necessary after the
provider has completed an initial nursing assessment;]
[(8)] (7)
(text unchanged)
[(9)] (8) Services are received by the
participant as documented by the signature of the participant or the
participant’s representative on the nursing provider’s official form; [and]
[(10) Participant has at least one caregiver willing and
able to accept responsibility for the participant’s care when the nurse, CNA,
or HHA is not available.]
(9) Services are determined medically necessary for a
participant after the provider has completed an initial nursing assessment that
reflects the participant’s need for an awake and alert caregiver;
(10) Participant has at least one caregiver willing and able to
accept responsibility for the participant’s care when the nurse, CNA, or HHA is
not available;
(11) Caregiver provides documentation of each of the following
when applicable:
(a) The caregiver’s work schedule along with commuting times;
(b) The caregiver’s school attendance as defined in Regulation
.02 of this chapter along with commuting times; and
(c) Emergency circumstances, as determined by the Department,
including but not limited to the inability of the primary caregiver to provide
care due to hospitalization or an acute debilitating illness for up to a 60-day
period; and
(12) Services are provided only in the absence of the willing
and able caregiver during sleeping hours and during the times documented in §A(11)
of this regulation.
.05 Limitations.
A. (text unchanged)
[B. Services to substitute for care ordinarily rendered by
the caregiver or caregivers shall be considered medically necessary:
(1) When the services meet the requirements of Regulation .04A of
this chapter; and
(2) When the:
(a) Participant requires an awake and alert caregiver at all times;
(b) Caregiver or caregivers provide documentation, including work
schedule, commuting times, and school attendance records as defined in
Regulation .01 of this chapter, that substitute care is necessary to allow
employment or school attendance; or
(c) Caregiver or caregivers provide documentation of emergency
circumstances, as determined by the Department, including but not limited to
the inability of the primary caregiver to provide care due to hospitalization
or an acute debilitating illness for up to a 60-day period.]
[C.] B.—[E.]
D. (text unchanged)
LAURA HERRERA
SCOTT
Secretary of Health
Subtitle 09 MEDICAL
CARE PROGRAMS
10.09.56 Home and
Community-Based Services Waiver for Children with Autism Spectrum Disorder
Authority: Health-General Article, §§2-104(b), 15-103, 15-105, and
15-130, Annotated Code of Maryland
Notice of Proposed Action
[23-341-P]
The Secretary of Health proposes to amend Regulations .02, .04,
.10, .14—.17, .19, .21, and .22 under COMAR
10.09.56 Home and Community-Based Services Waiver for Children with Autism
Spectrum Disorder.
Statement of Purpose
The purpose of this action is to update regulations to reflect
changes to the approved CMS waiver document. Additionally, the proposed action
will update the fee schedule for autism waiver providers, effective July 1,
2023.
Estimate of Economic Impact
The proposed action has no economic impact.
Economic Impact on Small Businesses
The proposed action has minimal or no economic impact on small
businesses.
Impact on Individuals with Disabilities
The proposed action has no impact on individuals with
disabilities.
Opportunity for Public Comment
Comments may be sent to Jourdan Green, Director, Office of
Regulation and Policy Coordination, Maryland Department of Health, 201 West Preston
Street, Room 512, Baltimore, MD 21201, or call 410-767-6499 (TTY 800-735-2258),
or email to mdh.regs@maryland.gov. Comments will be accepted through March 25,
2024. A public hearing has not been scheduled.
.02 Participant Eligibility.
A. Medical Eligibility for the Autism Waiver.
(1) To be medically eligible for the services covered under the
chapter, an applicant shall be certified by the licensed psychologist [or certified school psychologist, who
is a member of the participant’s multidisciplinary team and is employed by the
local Infants and Toddlers Program, the local school system, the Program, or
the Program’s designee], licensed social worker, licensed clinical
professional counselor, certified school psychologist, or approved service
coordinator employed or contracted by the local lead agency, the local
education agency, the State, or the State’s designee to need ICF-IID level
of care using the standardized process for determination of eligibility for
level of care in an ICF-IID.
(2) Every 12 months, or more frequently if determined necessary by
the service coordinator or multidisciplinary team due to a significant change
in the participant’s condition or needs, a participant’s medical need for
ICF-IID level of care shall be reevaluated by the licensed psychologist [or certified school psychologist on
the multidisciplinary team], licensed social worker, licensed clinical
professional counselor, certified school psychologist, or approved service
coordinator employed or contracted by the local lead agency, the local
education agency, the State, or the State’s designee, as part of the
multidisciplinary team process and using the form for determination of
eligibility for level of care in an ICF-IID.
(3) The form certifying ICF-IID level of care shall be signed by the:
(a) (text unchanged)
(b) Team's licensed psychologist [or certified school psychologist], licensed social worker, licensed clinical professional
counselor, certified school psychologist, or approved service coordinator
employed or contracted by the local lead agency, the local education agency,
the State, or the State’s designee.
B.—D. (text unchanged)
.04 Conditions for Participation — General.
To provide Autism Waiver services, the provider:
A.—D. (text unchanged)
E. Shall assure that direct care workers who render services under
this chapter:
(1) [Have at least a
high school degree or GED, including equivalency review by an accredited agency
for credentials obtained outside of the United States] Meet the minimum age requirement, set forth
by service, before rendering services to any Autism
Waiver participant;
(2)—(6) (text unchanged)
F.—K. (text unchanged)
L. If an agency, shall require supervisory and direct care
employees of provider agencies to:
(1) (text unchanged)
(2) Request the Department of Public Safety and Correctional
Services to send the child care criminal history report to the [agency of employment] Department’s
Division of Community Long Term Care; and
(3) (text unchanged)
M. If an agency, shall:
(1) (text unchanged)
(2) [Maintain the
original child care criminal history report for all agency and contracted
employees as well as any updated criminal history reports from the Department
of Public Safety and Correctional Services in the employee's personnel record] Request
clearance from the Department for all applicants prior to an offer of
employment; and
(3) (text unchanged)
N. If self-employed, shall:
(1) (text unchanged)
(2) Request the Department of Public Safety and Correctional
Services to send the child care criminal history report to the [Department] Department’s Division of
Community Long Term Care;
(3)—(5) (text unchanged)
O.—II. (text unchanged)
.10 Specific Conditions for Participation — Adult Life Planning
Services.
A. To provide the services covered under Regulation .19 of this
chapter, the provider shall:
(1) Be an individual with a [Master's
Degree in Human Services] bachelor’s degree; and
(2) Have [5] 3
years of full-time experience serving adults with autism disabilities.
B.—E. (text unchanged)
.14 Covered Services — Therapeutic Integration Services.
A. (text unchanged)
B. [A] For dates of service before July 1, 2023, a
unit of service is a 30-minute increment of service rendered to a participant
by a qualified provider in the community setting.
C. For dates of service on or after July 1, 2023, a unit of
service is a 15-minute increment of service rendered to a participant by a
qualified provider in the community setting.
.14-1 Covered Services — Intensive Therapeutic Integration
Services.
A. (text unchanged)
B. [A] For dates of service before July 1, 2023, a
unit of service is a 30-minute increment of service rendered to a participant
by a qualified provider in the community setting.
C. For dates of service on or after July 1, 2023, a unit of
service is a 15-minute increment of service rendered to a participant by a
qualified provider in the community setting.
.15 Covered Services — Intensive Individual Support Services.
A.—C. (text unchanged)
D. [A] For
dates of service before July 1, 2023, a unit of service is a 30-minute
increment of service rendered to a participant by a qualified provider in the participant’s home or a community
setting.
E. For dates of service on or after July 1, 2023, a unit of
service is a 15-minute increment of service rendered to a participant by a
qualified provider in the participant’s home or a community setting.
.16 Covered Services — Respite Care.
A.—B. (text unchanged)
C. [A] For dates of service before July 1, 2023, a
unit of service is a 30-minute increment of service rendered to a participant
by a qualified provider in the participant’s home or a community setting.
D. For dates of service on or after July 1, 2023, a unit of
service is a 15-minute increment of service rendered to a participant by a
qualified provider in the participant’s home or a community setting.
.17 Covered Services — Family Consultation.
A.—D. (text unchanged)
E. [A] For dates of service before July 1, 2023, a
unit of service is a 30-minute increment of service rendered to a participant
by a qualified provider in the participant’s home or a community setting.
F. For dates of service on or after July 1, 2023, a unit of
service is a 15-minute increment of service rendered to a participant by a
qualified provider in the participant’s home or a community setting.
.19 Covered Services — Adult Life Planning Services.
A. (text unchanged)
B. Adult life planning services shall:
(1)—(2) (text unchanged)
(3) Be provided only to participants [age 16] 14 years old or older.
C. [A] For dates of service before July 1, 2023, a
unit of service is a 30-minute increment of service rendered to a participant
by a qualified provider in the participant’s home or a community setting.
D. For dates of service on or after July 1, 2023, a unit of
service is a 15-minute increment of service rendered to a participant by a
qualified provider in the participant’s home or a community setting.
.21 Limitations.
A.—D. (text unchanged)
E. Therapeutic integration and intensive therapeutic integration
services may include transportation time when at least 2 units of service have
been provided on-site, and the maximum units of service billed may not exceed:
(1) For dates of service before July 1, 2023, 24 units;
or
(2) For dates of service on or after July 1, 2023, 12 units.
F.—G. (text unchanged)
H. The Program may reimburse for a participant not more than:
(1) For dates of service before July 1, 2023:
[(1)] (a) [One] 1 unit
per date of service for residential habilitation services at either the regular
or intensive level;
[(2)] (b)—[(3)] (c) (text
unchanged)
[(4)] (d) 12 units or fewer than [two]
2 units of therapeutic integration or intensive therapeutic integration
services for a date of service;
[(5)] (e)—[(13)] (m) (text
unchanged)
[(14)] (n) 15 units of retainer payment per
calendar year at either the regular or intensive level when the participant is
absent from the residential habilitation program for the purposes of family
visitation, hospitalization, or other overnight stays[.]; or
(2) For dates of service on or after July 1, 2023:
(a) 1 unit per date of service for residential habilitation
services at either the regular or the intensive level;
(b) 80 units of therapeutic integration services per week;
(c) 60 units of intensive therapeutic integration services per
week;
(d) 24 units or fewer than 2 units of therapeutic integration or
intensive therapeutic integration services for a date of service;
(e) 160 units of intensive individual support services per week;
(f) 96 units of respite care for a date of service;
(g) 1344 units of respite care per State fiscal year;
(h) 24 units of family consultation for a date of service;
(i) 160 units of family consultation per State fiscal year;
(j) A total of $2,104 for environmental accessibility
adaptations over a 36-month period;
(k) 32 units of intensive individual support services per day;
(l) 80 units of adult life planning services per State fiscal
year, for participants 14 years old or older;
(m) 16 units of adult life planning services per date of
service, for participants 14 years old or older; and
(n) 15 units of retainer payment per calendar year at either the
regular or intensive level when the participant is absent from the residential
habilitation program for the purposes of family visitation, hospitalization, or
other overnight stays.
I.—L. (text unchanged)
.22 Payment Procedures.
A.—D. (text unchanged)
E. Rates.
(1)—(3) (text unchanged)
(4) Effective July 1, 2023, the Program shall pay according to
the following fee-for-service schedule:
(a) Residential habilitation services and retainer payments
reimbursed at one of the following all-inclusive, maximum rates for a
participant:
(i) $283.69 per unit for the regular level of service; or
(ii) $567.45 per unit for the intensive level of service.
(b) Therapeutic integration services reimbursed at the maximum
rate of $8.5950 per unit.
(c) Intensive therapeutic integration services reimbursed at the
maximum rate of $10.7450 per unit.
(d) Intensive individual support services reimbursed at the
maximum rate of $10.7450 per unit.
(e) Respite care reimbursed at the maximum rate of $8.4000 per
unit.
(f) Family consultation reimbursed at the maximum rate of
$35.2750 per unit.
(g) Adult life planning services reimbursed at the maximum rate
of $35.2750 per unit.
(h) Environmental accessibility adaptions reimbursed at the
maximum rate of $2,104 per 36-month period amount billed by the provider, which
shall be the lesser of the:
(i) Amount authorized by the State Department of Education; or
(ii) Actual cost of the job.
LAURA HERRERA SCOTT
Secretary of Health
Subtitle 09 MEDICAL
CARE PROGRAMS
10.09.69 Maryland Medicaid
Managed Care Program: Rare and Expensive Case Management
Authority: Health-General Article, §§2-104(b), 15-103(b)(4)(i),
and 15-105, Annotated Code of Maryland
Notice of Proposed Action
[23-349-P]
The Secretary of Health proposes to amend Regulations .02, .11,
and .12 under COMAR 10.09.69 Maryland Medicaid Managed Care Program:
Rare and Expensive Case Management.
Statement of Purpose
The purpose of this action is to
clarify covered optional private duty nursing (PDN) services when
provided to participants eligible for rare and expensive case management (REM)
services.
Estimate of Economic Impact
The proposed action has no economic impact.
Economic Impact on Small Businesses
The proposed action has minimal or no economic impact on small
businesses.
Impact on Individuals with Disabilities
The proposed action has an impact on individuals with disabilities
as follows:
Individuals with disabilities receive services provided under this
chapter and will benefit to the extent that clarification of the application of
the current regulations improve transparency to the medically complex
population served.
Opportunity for Public Comment
Comments may be sent to Jourdan Green, Director, Office of
Regulation and Policy Coordination, Maryland Department of Health, 201 West
Preston Street, Room 512, Baltimore, MD 21201, or call 410-767-6499 (TTY
800-735-2258), or email to mdh.regs@maryland.gov. Comments will be accepted
through March 25, 2024. A public hearing has not been scheduled.
.02 Definitions.
A. (text unchanged)
B. Terms Defined.
(1)—(22) (text unchanged)
(23) “Home” [has the meaning stated in COMAR 10.09.53.01]
means the place of residence, occupied by the participant, other than a
residence or facility where nursing services are included in the living
arrangement by regulation or statute, or otherwise provided for payment.
(24)—(55) (text unchanged)
(56) “REM optional services” means the services listed in
Regulations [.09] .10
and [.10] .11 of this
chapter, which meet the general requirements under Regulation [.08]
.09 of this chapter.
(56)—(58) (text unchanged)
(59) “School” means courses or classes for the acquisition of a
General Education Diploma, a high school diploma, an associate degree, or a
first-time bachelor’s degree.
[(59)] (60)—[(60)]
(61) (text unchanged)
.11 Covered Optional Services — Private Duty Nursing, Certified
Nursing Assistant, Certified Nursing Assistant Certified as a Certified
Medication Technician, Home Health Aide and Home Health Aide Certified as a
Certified Medication Technician.
A. The Program shall cover shift nursing services provided by an RN
or LPN when:
(1)—(3) (text unchanged)
(4) Services are [rendered in accordance with COMAR
10.09.53;] determined medically necessary for a participant after the
provider has completed an initial nursing assessment that reflects the
participant’s need for an awake and alert caregiver;
(5) The participant has at least one caregiver willing and able
to accept responsibility for the participant’s care when the nurse, CNA, or HHA
is not available;
(6) The caregiver provides documentation of each of the
following when applicable:
(a) The caregiver’s work schedule along with commuting times;
(b) The caregiver’s school attendance as defined in Regulation
.02 of this chapter along with commuting times; and
(c) Emergency circumstances, as determined by the Department,
including but not limited to the inability of the primary caregiver to provide
care due to hospitalization or an acute debilitating illness for up to a 60-day
period;
(7) Services are provided only in the absence of the willing and
able caregiver during the sleeping hours and during the times documented in §A(6)
of this regulation;
[(5)] (8)—[(7)]
(10) (text unchanged)
[(8)] (11) Services are rendered by an RN or
an LPN who is certified in cardiopulmonary resuscitation and the certification
is renewed every 2 years; and
[(9) Services are preauthorized in accordance with the
criteria set forth in COMAR 10.09.53; and]
[(10)] (12)
(text unchanged)
B.—C. (text unchanged)
.12 Limitations.
A.—B. (text unchanged)
C. The REM program does not cover the following:
(1)—(7) (text unchanged)
(8) Speech, language, or occupational therapy services rendered in
a classroom setting; [and]
(9) Shift private duty nursing, CNA, CNA-CMT, HHA, or HHA-CMT
services ordered by a physician assistant[.];
(10) Custodial services;
(11) Services provided to a participant in a:
(a) Hospital;
(b) Residential treatment center;
(c) Intermediate care facility for individuals with intellectual
disabilities; or
(d) Residence or facility where nursing services are included in
the living arrangement by regulation or statute, or otherwise provided for
payment;
(12) Services not directly related to the participant’s plan of
care;
(13) Services described in the plan of care whenever a major
change occurs in the participant's medical condition or skilled nursing care
needs that indicates such services are no longer necessary;
(14) Services which are not initially ordered before the start
of care and renewed every 60 days by the participant’s primary medical
provider;
(15) Services provided by a nurse, CNA, or HHA who does not
possess a valid, current, and nontemporary nursing license or certifications to
provide services in the jurisdiction in which nursing services are rendered;
(16) Services provided by a nurse, CNA, or HHA who does not have
a current cardiopulmonary resuscitation (CPR) certification for the period
during which the services are rendered;
(17) Direct payment for supervisory visits that do not meet
acceptable standards of practice in accordance with COMAR 10.27.09, 10.27.10,
and 10.27.11;
(18) Services rendered to a participant by a nurse, CNA, or HHA
in the assigned staff’s home;
(19) Services not documented; and
(20) Respite services.
D. The Program shall only cover one-to-one nursing when a
participant’s condition requires that level of service and shared services are
not an option.
E. The Program shall only cover nursing services ordered by an
individual who is enrolled as a provider in the Program with an active status
on the date of service.
LAURA HERRERA SCOTT
Secretary of Health
Subtitle 09 MEDICAL
CARE PROGRAMS
10.09.89 1915(i) Intensive
Behavioral Health Services for Children, Youth, and Families
Authority: Health-General Article, §§2-104(b) and 15-105,
Annotated Code of Maryland
Notice of Proposed Action
[23-346-P]
The Secretary of Health proposes to amend Regulation .14
under COMAR 10.09.89 1915(i) Intensive Behavioral Health Services for
Children, Youth, and Families.
Statement of Purpose
The purpose of this action is to update the listed provider
reimbursement rate to the 3 percent increased rate, effective for Fiscal Year
2024, pursuant to Ch. 101 (H.B. 200), Acts of 2023, Fiscal Year 2024 Budget.
Estimate of Economic Impact
I. Summary of Economic Impact. The budget for Fiscal Year
2024 includes a 3 percent rate increase. The total fiscal impact for this rate
increase on 1915(i) services is $982.35.
II. Types of Economic Impact.
|
Impacted Entity
|
Revenue
(R+/R-)
Expenditure
(E+/E-)
|
Magnitude
|
|
A. On issuing agency:
|
|
|
|
Maryland Department of Health
|
(E+)
|
$982.35
|
|
B. On other State agencies:
|
NONE
|
|
|
C. On local governments:
|
NONE
|
|
|
|
|
|
|
|
Benefit
(+)
Cost
(-)
|
Magnitude
|
|
D. On regulated industries or
trade groups:
|
|
|
|
Maryland Medicaid 1915(i)
providers
|
(+)
|
$982.35
|
|
E. On other industries or
trade groups:
|
NONE
|
|
|
F. Direct and indirect
effects on public:
|
NONE
|
|
III. Assumptions. (Identified by Impact Letter and Number
from Section II.)
A. This amount assumes:
(1) The estimated economic impact of the proposed action is based
on Fiscal Year 2023 program expenditures and projected expenditure for Fiscal
Year 2024.
(2) Annual 1915(i) service utilization is expected to remain
consistent between Fiscal Year 2023 and Fiscal Year 2024.
(3) The Fiscal Year 2024 impact is the difference in projected
expenditures between Fiscal Year 2023 ($32,745.10) and Fiscal Year 2024
($33,727.45).
(4) This amount ($982.53) is subject to a 66.49 percent federal
match, utilizing 66.49 percent funds ($653.17) and 33.51 percent general funds
($329.19).
D. See A above.
Economic Impact on Small Businesses
The proposed action has a meaningful economic impact on small
businesses. An analysis of this economic impact follows:
Providers enrolled as Maryland Medicaid 1915(i) providers will
benefit from increased reimbursement.
Impact on Individuals with Disabilities
The proposed action has no impact on individuals with
disabilities.
Opportunity for Public Comment
Comments may be sent to Jourdan Green, Director, Office of
Regulation and Policy Coordination, Maryland Department of Health, 201 West
Preston Street, Room 512, Baltimore, MD 21201, or call 410-767-6499 (TTY
800-735-2258), or email to mdh.regs@maryland.gov. Comments will be accepted
through March 25, 2024. A public hearing has not been scheduled.
.14 Payment Procedures.
A.—D. (text unchanged)
E. Family peer support services as described in Regulation .09 of
this chapter shall be reimbursed at the following rates:
(1) For dates of service from [January 1, 2021] July
1, 2022 through [October 31, 2021] June 30, 2023:
(a) [$19.16] $21.65 per 15-minute unit for
face-to-face services; or
(b) [$9.57] $10.82 per 15-minute unit for
telephonic or other non-face-to-face activities.
[(2) For dates of service from November 1, 2021 through June
30, 2022:
(a) $20.19 per 15-minute unit for face-to-face services; or
(b) $10.09 per 15-minute unit for telephonic or other
non-face-to-face activities.]
[(3)] (2) Effective July 1, [2022] 2023:
(a) [$21.65] $22.30 per 15-minute unit for
face-to-face services; or
(b) [$10.82] $11.14 per 15-minute unit for
telephonic or other non-face-to-face activities.
F. Respite services as described in Regulation .10 of this chapter
shall be reimbursed at the following rates:
(1) For dates of service from [January 1, 2021] July
1, 2022 through [October 31, 2021] June 30, 2023:
(a) [$30.18] $34.12 per 1-hour unit of service
for community-based respite services; or
(b) [$239.26] $270.46 per unit of out-of-home
respite care.
[(2) For dates of service from November 1, 2021 through June
30, 2022:
(a) $31.81 per 1-hour unit of service for community-based respite
services; or
(b) $252.18 per unit of out-of-home respite care.]
[(3)] (2) Effective July 1, [2022] 2023:
(a) [$34.12] $35.14 per 1-hour unit of service
for community-based respite services; or
(b) [$270.46] $278.57 per unit of out-of-home
respite care.
G. Expressive and experiential behavioral services as described in
Regulation .11 of this chapter, when provided by a licensed mental health
professional, shall be reimbursed at the following rates:
(1) For dates of service from [January 1, 2021] July
1, 2022 through [October 31, 2021] June 30, 2023:
(a) For individual therapy:
(i) [$82.07] $92.77 per 45—50-minute session;
or
(ii) [$107.52] $121.55 per 75—80-minute
session; and
(b) For group therapy:
(i) [$32.62] $36.87 per 45—60-minute session;
or
(ii) [$42.42] $47.95 per prolonged
(75—90-minute) session.
[(2) For dates of service from November 1, 2021 through June
30, 2022:
(a) For individual therapy:
(i) $86.50 per 45—50-minute session; or
(ii) $113.33 per 75—80-minute session; and
(b) For group therapy:
(i) $34.38 per 45—60-minute session; or
(ii) $44.71 per prolonged (75—90-minute) session.]
[(3)] (2) Effective July 1, [2022] 2023:
(a) For individual therapy:
(i) [$92.77] $95.55 per 45—50-minute session;
or
(ii) [$121.55] $125.20 per 75—80-minute
session; and
(b) For group therapy:
(i) [$36.87] $37.98 per 45—60-minute session;
or
(ii) [$47.95] $49.39 per prolonged
(75—90-minute) session.
H. Expressive and experiential behavioral services as described in
Regulation .11 of this chapter, when provided by a non-licensed mental health
professional, shall be reimbursed at the following rates:
(1) For dates of service from [January 1, 2021] July
1, 2022 through [October 31, 2021] June 30, 2023:
(a) For individual therapy:
(i) [$74.60] $84.33 per 45-minute session; or
(ii) [$96.99] $109.64 per 75—80-minute
session; and
(b) For group therapy:
(i) [$28.99] $32.78 per 45—60-minute session;
or
(ii) [$37.68] $42.59 per prolonged
(75—90-minute) session.
[(2) For dates of service from November 1, 2021 through June
30, 2022:
(a) For individual therapy:
(i) $78.68 per 45-minute session; or
(ii) $102.23 per 75—80-minute session; and
(b) For group therapy:
(i) $30.56 per 45—60-minute session; or
(ii) $39.71 per prolonged (75—90-minute) session.]
[(3)] (2) Effective July 1, [2022] 2023:
(a) For individual therapy:
(i) [$84.33] $86.86 per 45-minute session; or
(ii) [$109.64] $112.93 per 75—80-minute
session; and
(b) For group therapy:
(i) [$32.78] $33.76 per 45—60-minute session;
or
(ii) [$42.59] $43.87 per prolonged
(75—90-minute) session.
I. Intensive in-home services as described in Regulation .12 of
this chapter shall be reimbursed at the following rates:
(1) For dates of service from [January 1, 2021] July
1, 2022 through [October 31, 2021] June 30, 2023:
(a) [$298.60] $337.54 per week of service for
EBP-IIHS providers; or
(b) [$236.89] $267.78 per week of service for
non-EBP IIHS providers.
[(2) For dates of service from November 1, 2021 through June
30, 2022:
(a) $314.72 per week of service for EBP-IIHS providers; or
(b) $249.68 per week of service for non-EBP IIHS providers.]
[(3)] (2) Effective July 1, [2022] 2023:
(a) [$337.54] $347.67 per week of service for
EBP-IIHS providers; or
(b) [$267.78] $275.81 per week of service for
non-EBP IIHS providers.
LAURA HERRERA
SCOTT
Secretary of Health
Subtitle 19 DANGEROUS DEVICES AND
SUBSTANCES
10.19.03 Controlled Dangerous
Substances
Authority: Criminal Law Article, Title 5; Health-General Article,
§21-220; Annotated Code of Maryland
Notice of Proposed Action
[23-335-P]
The Secretary of Health proposes to repeal existing Regulations .01—.13 and adopt new Regulations .01—.20 under COMAR 10.19.03 Controlled Dangerous Substances.
Statement of Purpose
The purpose of this action is to evaluate COMAR 10.19.03 for the
purpose of satisfying the requirement of State Government Article,
§§10-130—10-139, Annotated Code of Maryland, to update the regulations per
statutory changes, and to reorganize and clarify existing language.
Estimate of Economic Impact
The proposed action has no economic impact.
Economic Impact on Small Businesses
The proposed action has minimal or no economic impact on small
businesses.
Impact on Individuals with Disabilities
The proposed action has no impact on individuals with
disabilities.
Opportunity for Public Comment
Comments may be sent to Jourdan Green, Director, Office of
Regulation and Policy Coordination, Maryland Department of Health, 201 West
Preston Street, Room 512, Baltimore, MD 21201, or call 410-767-6499 (TTY
800-735-2258), or email to mdh.regs@maryland.gov. Comments will be accepted
through March 25, 2024. A public hearing has not been scheduled.
.01 Scope and Purpose.
A. The Secretary
of the Maryland Department of Health recognizes that the public interest
requires control over the use of controlled substances in the legitimate
manufacturing, distributing, dispensing, storing, and ordering of controlled
substances.
B. This chapter
applies to every registrant who manufactures, distributes, dispenses, stores,
and orders any controlled substances within the State of Maryland, unless
exempted from registration by Criminal Law Article, §5-301(d), Annotated Code
of Maryland, or 21 CFR §§1301.22 — 1301.24.
C. In the event of
conflict between a provision of the Code of Federal Regulations referenced
under a provision of this chapter and the provision of this chapter, the
registrant shall comply with the more stringent provision.
.02 Incorporation by Reference.
In this chapter,
21 CFR §§1300 —1317, as amended, are incorporated by reference.
.03 Definitions.
A. Applicability
to Chapter. In this chapter, the terms defined in §B of this regulation have
the meanings indicated. In addition, and unless otherwise provided in this
chapter, the definitions appearing in Criminal Law Article, §5-101, Annotated
Code of Maryland, shall also apply to this chapter.
B. Terms Defined.
(1) “Act” means
the Maryland Controlled Dangerous Substances Act, Criminal Law Article, §§5-101—5-1101, Annotated Code of Maryland.
(2)
“Administrative probable cause” means a valid public interest, sufficient to
justify an administrative warrant, allowing the inspection of the area,
premises, or building to enforce this chapter.
(3) “Agent” means
a person who acts on behalf of the Department.
(4) “Authorized
provider” or “provider” means a:
(a) Person
licensed, registered, or otherwise allowed to dispense, order, and store
controlled substances;
(b) Pharmacy,
laboratory, hospital, or other institution licensed, registered, or otherwise
allowed to manufacture, distribute, dispense, store, and order controlled
substances; or
(c) Person
licensed, registered, or otherwise allowed to conduct research on a controlled
substance in the State in the course of professional practice or research.
(5) “Board” means
the Maryland Boards of Physicians, Podiatry, Dental Examiners, Nursing, and
Veterinary Medicine.
(6) “Chemical
analysis” means the act of decomposing a substance into its constituent
elements.
(7) “Civil fine”
means a fine assessed by the Department against an authorized provider,
including establishments.
(8) Commercial
Container.
(a) “Commercial
container” means any bottle, jar, tube, ampule, or other receptacle in which a
substance is held for distribution or dispensing to an ultimate user.
(b) “Commercial
container” includes any box or package in which a receptacle is held for
distribution or dispensing to an ultimate user.
(c) “Commercial
container” does not include any package liner, package insert, or other
material kept with or within a commercial container, or any carton, crate,
drum, or other package in which commercial containers are stored or are used
for shipment of controlled substances.
(9) Controlled
Premises.
(a) “Controlled
premises” means places where original or other records or documents required
under the Act are kept or required to be kept.
(b) “Controlled
premises” includes factories, warehouses, other establishments, and
conveyances, where persons registered under the Act or exempt from registration
under the Act may lawfully hold, manufacture, distribute, dispense, administer,
or otherwise dispose of controlled substances.
(10) “Department”
means the Maryland Department of Health.
(11) “Disciplinary
action” includes but is not limited to a penalty, reprimand, restriction,
suspension, or revocation of a license.
(12) “Federal Act”
means the Controlled Substance Act (84 Stat. 1242; 21 U.S.C. 801) or the
Controlled Substance Import and Export Act (84 Stat. 1285; 21 U.S.C. 951).
(13) “Fee exempt”
means an authorized provider that is required to register with the Department
but is not required to pay the registration fee.
(14) “In good
standing” means having a professional license that is not subject to any form
of sanction by the Board or Criminal Law.
(15) “Inspector”
means an officer or employee of the Department authorized by the Secretary to
make inspections under the Act.
(16) “Labeling”
means all labels and other written, printed, or graphic matter on any
controlled substance or any of its commercial containers or wrappers
accompanying controlled substances.
(17) “License” has
the same meaning as State Government Article, §10-226, Annotated Code of
Maryland.
(18) Manufacture.
(a) “Manufacture”
means:
(i) Producing,
preparation, propagation, compounding, or processing of a drug or other
substance;
(ii) Packaging or
repackaging of the substance; or
(iii) Labeling or
re-labeling of the commercial container of the substance.
(b) “Manufacture” does not include the activities of a provider
who, as an incident to the provider’s administration or dispensing the
substance in the course of the provider’s professional practice, prepares,
compounds, packages, or labels the substance.
(19) “Manufacturer” means a person who manufactures a drug or
other substance under:
(a) A registration as a manufacturer; or
(b) Authority of registration as a researcher or chemical
analyst.
(20) “Office of Controlled Substances Administration (OCSA)”
refers to the division within the Maryland Department of Health responsible for
administering the enforcement of Criminal Law Article, Title 5, Annotated Code
of Maryland.
(21) “Penalty” means monetary penalty, including but not limited
to a fine.
(22) “Person” means:
(a) An authorized provider;
(b) The responsible party listed on the application licensed by
the DEA; or
(c) The responsible party listed on the application for an
establishment not licensed by the DEA.
(23) “Peyote” means the controlled substance in Schedule I under
Criminal Law Article, §5-402, Annotated Code of Maryland. This schedule I
listing does not apply to the nondrug use of peyote in bona fide religious
ceremonies of the Native American Church, and members of the Native American
Church using peyote in this manner are exempt from registration.
(24) “Pharmacist” means:
(a) A pharmacist licensed in the State or otherwise authorized
to dispense controlled substances; or
(b) An individual, for example, a pharmacist intern, authorized
by a state to dispense controlled substances under the supervision of a
pharmacist licensed by that state.
(25) Prescription.
(a) “Prescription” means an order for medication that is
dispensed to or for an ultimate user.
(b) “Prescription” does not include an order for medication
which is dispensed for immediate administration to the ultimate user, such as
an order to dispense a drug to a bed patient for immediate administration in a
hospital.
(26) “Registration” means initial registration or subsequent
re-registration with OCSA.
(27) “Secretary” means the Secretary of the Maryland Department
of Health.
(28) “Sufficient application” means an application that is
completed in full and has provided all of the information required by the
Department.
.04 Registration: Requirements and
Qualifications.
A. Except for
military and federal employees working within the scope of federal employment,
a person shall register with the Department and obtain a registration
certificate before the person:
(1) Manufactures,
distributes, dispenses, stores, and orders controlled substances;
(2) Conducts
research or instructional activities with controlled substances listed in
Schedules II through V of Criminal Law Article, §§5-403—5-406, Annotated Code
of Maryland;
(3) Conducts
research or instructional activities with a controlled substance listed in
Schedule I of Criminal Law Article, §5-402, Annotated Code of Maryland, with
prior registration from the DEA;
(4) Conducts a
chemical analysis with controlled substances listed in any schedule;
(5) Installs and
maintains an automated dispensing machine containing controlled substances; or
(6) Manufactures
peyote for, or distributes peyote to, the Native American Church for bona fide
religious ceremonies of the Native American Church.
B. An authorized
provider shall:
(1) Apply for a
registration certificate on a form made available by the Department to:
(a) Obtain one
registration for all locations that a provider prescribes only and no
controlled substances are stored; or
(b) Obtain a
single registration for each separate location where the provider prescribes,
dispenses, orders, and stores controlled substances;
(2) Have a
professional license in good standing;
(3) Be of good
moral character;
(4) Have no
convictions for crimes of moral turpitude;
(5) Be 18 years of
age or older;
(6) Be registered
with the Prescription Drug Monitoring Program if the authorized provider is
also a prescriber as defined under Health-General Article, §21-2A-04.1,
Annotated Code of Maryland; and
(7) Except for
researchers, have completed 2 continuing education credit hours:
(a) Prior to
applying for an initial controlled substance registration occurring on or after
October 1, 2018; or
(b) Prior to the
first renewal of a controlled substance registration occurring on or after
October 1, 2018.
C. Continuing
Education.
(1) The continuing
education shall:
(a) Be accredited
by the Accreditation Council for Continuing Medical Education; or
(b) Meet the
requirements for approval by the authorized providers professional board.
(2) The continuing
education shall pertain to the prescribing or dispensing of controlled
substances.
(3) Authorized
providers shall meet the requirements of §§B and C of this regulation upon the
completion of the 2 credit hours of continuing education.
(4) Recent
graduates meet the requirements of §§B and C of this regulation upon initial
application if two academic course credits were issued relating to controlled
substances.
D. An
establishment shall:
(1) Apply for a
registration certificate on a form made available by the Department;
(2) Obtain one
registration for each separate place of business, professional practice, or
location where controlled substances are:
(a) Ordered;
(b) Purchased;
(c) Administered;
(d) Delivered to
the ultimate user;
(e) Stored;
(f) Manufactured;
or
(g) Distributed;
and
(3) Have a
professional or occupational license in good standing to perform any of the
following functions related to controlled substances:
(a) Manufacture;
(b) Distribute;
(c) Deliver to the
ultimate user;
(d) Administer;
(e) Store;
(f) Order;
(g) Prescribe;
(h) Package;
(i) Label; or
(j) Compound.
.05 Registration: Application.
A. A person shall
complete an application to be considered for a registration certificate.
B. The applicant
shall:
(1) Submit a
completed application made available by the Department;
(2) Except for fee
exempt applicants, pay the appropriate fee as set forth in Regulation .06 of
this chapter;
(3) Unless exempt
from payment of a registration fee, apply for and obtain a registration
certificate;
(4) Provide an
explanation for any not-applicable responses on the applications;
(5) Notify the
Department of any criminal convictions related to controlled substances;
(6) Notify the
Department of any actions taken on the applicant’s professional license;
(7) Have the
application and accompanying documents required for registration signed by the:
(a) Applicant, if
an individual;
(b) General
partner, if the applicant is a partnership; or
(c) Officer
responsible for the applicant, if the applicant is a corporation or other
entity; and
(8) Provide
additional documentation required by the Department pertinent to the
registration, to:
(a) Clarify
application information; and
(b) Determine if
the applicant meets the requirements of this chapter.
C. New
applications that are unable to be processed 6 months from the payment deposit
date:
(1) May be
determined invalid;
(2) Will not be
issued a refund, unless:
(a) The payment is
a duplicate; or
(b) Approved by
the Department; and
(3) Will require a
new application to be resubmitted.
D. Renewal
applications that are unable to be processed 45 days from the payment deposit
date:
(1) May be
determined invalid;
(2) Will not be
issued a refund, unless:
(a) The payment is
a duplicate; or
(b) Approved by
the Department; and
(3) Will require a
new renewal application to be submitted.
.06 Registration: Fees.
A. An authorized
provider or establishment required to obtain and maintain a registration
certificate as set forth under this chapter shall pay the appropriate fees as
follows:
(1) New
registration certificate — $120;
(2) Renewal
registration certificate — $120;
(3) Amend a
registration certificate — $50;
(4) Change of
ownership fee — $144;
(5) Duplicate
registration certificate — $30; and
(6) Reinstatement
— $50.00.
B. Applicants
exempt from registration fees include:
(1) Except for
contractual positions, an employee of a hospital, clinic, institution,
facility, or unit operated by a local or State government agency;
(2) An agency,
excluding a State agency and its employees, for which the State is responsible
for payment of the fee, provided that the exemption is approved by the
Secretary; or
(3) A person
included in 21 CFR §§1301.21.
C. Refunds and
Credits.
(1) The Secretary
may not issue a credit towards or refund any registration fees to an applicant,
including but not limited to, an applicant who:
(a) Withdraws a
registration application;
(b) Voluntarily or
involuntarily ceases to manufacture, distribute, dispense, order, and store a
controlled substances before a registration certificate expires; or
(c) Does not
complete the application as set forth in Regulation .05 of this chapter.
(2) The Secretary,
or the Secretary’s designee, shall review the refund request and determine if a
refund of any amount is appropriate.
D. A registrant
who has lost a registration certificate is required to pay a duplicate
registration fee as set forth in §A of this regulation.
E. The Department
may not issue a new or a renewed registration certificate until all required
fees are paid.
F. The
Department-issued registration certificate shall be readily available to any
agent or representative of the Secretary within the registered location.
.07 Registration: Term, Expiration, Renewal, and Reinstatement.
A. Term. The
Secretary may issue a registration certificate that is valid for not more than
3 years.
B. Expiration.
(1) A registration
certificate expires on the date shown on the certificate.
(2) The Secretary
shall notify the registrant that the registration will expire not less than 30
days before the expiration date shown on the certificate.
C. Renewal.
(1) A registration
for which a sufficient application for renewal was submitted:
(a) Shall be
received at least 2 weeks before the expiration; and
(b) Will remain in
effect until the Department takes a final action.
(2) The failure to
receive a renewal notice does not excuse a registrant from complying with this
chapter.
D. Reinstatement.
(1) A registrant
whose registration has been surrendered in lieu of further investigation,
revoked, suspended, or limited may not be eligible to request reinstatement for
a:
(a) 2-year period;
(b) Specified
period designated in a consent order from the Department; or
(c) Specified time
period determined by the Department.
(2) To apply for
reinstatement, a registrant shall:
(a) Complete and
submit an application on a form provided by the Department; and
(b) Pay a
reinstatement fee shown in Regulation .06 of this chapter.
.08 Registration Certificate: Assignment, Transfer, or Termination.
A. A registration certificate is not
transferable.
B. A registration
certificate becomes void when the registrant:
(1) Dies;
(2) Discontinues
business or professional practice in the State; or
(3) Changes
ownership, including when:
(a) Partners are
added or deleted from the partnership;
(b) There is a
change in the president or chief executive officer of the corporation; or
(c) There is a
change in the ownership of 10 percent or more of the outstanding shares in the
corporation.
C. A registrant
who discontinues business or professional practice in the State shall:
(1) Notify the
Secretary in writing;
(2) Surrender the
registrant’s current registration certificate; and
(3) Legally
transfer or dispose of any controlled substances relative to the registrant’s
operation.
D. A registrant who changes ownership of the
business or professional practice shall:
(1) Notify the
Secretary in writing at least 30 days before the change occurs;
(2) Complete and
submit a change of ownership application to the Department;
(3) Pay the
change-of-ownership fee in Regulation .06 of this chapter;
(4) Surrender the
registrant’s current registration certificate after the ownership change is
finalized; and
(5) Allow the
Department to inspect the business or professional practice of the new owners.
E. A registrant
who changes the location of a registrant’s place of business or professional
practice shall:
(1) Notify the
Department at least 30 days before there is a change in the location of the
registrant’s place of business or professional practice;
(2) Apply to amend
a current registration certificate; and
(3) Pay the fee to
amend a certificate in Regulation .06 of this chapter.
F. During the
3-year registration period, the registrant shall notify the Office of
Controlled Substances Administration within 30 days of the following:
(1) Any criminal
convictions;
(2) Any
disciplinary actions taken against the registrant’s professional or
occupational license; or
(3) Any
disciplinary actions taken against the registrant’s federal Drug Enforcement
Agency registration.
G. After an
application for registration has been filed, the Secretary may:
(1) Inspect the
place of business or professional practice described in the application; and
(2) Investigate
the applicant to determine whether the applicant meets the requirements of this
chapter.
H. The registrant
shall inform the Office of Controlled Substances Administration prior to any
address changes.
.09 Labeling and Packaging Requirements for Controlled Substances.
A registrant shall
comply with the provisions of 21 CFR §1302, as amended.
.10 Records and Reports Required of Registrants.
A. Pursuant to
Criminal Law Article, §5-306, Annotated Code of Maryland, a registrant shall
comply with the provisions of 21 CFR §1304, as amended.
B. Inventories
developed under the Federal Act dated May 1, 1971, and subsequent biennial
inventory dates are acceptable for purposes of the Maryland Act.
C. On the
effective date a substance is added to any schedule of controlled substances
pursuant to Criminal Law Article, §5-202, Annotated Code of Maryland,
registrants required to keep records, who possess that substance, shall take an
inventory of all stocks of that substance on hand. Thereafter, that substance shall be included
in each inventory made by the registrant.
D. Reports
required in this section shall be accessible to the representative of the
Secretary.
.11 Order Forms.
A registrant shall
comply with the provisions of 21 CFR 1305, as amended.
.12 Prescriptions.
A. Persons
Entitled to Issue Prescriptions.
(1) A prescription
for a controlled substance may be issued only by an authorized provider who is:
(a) Authorized to
prescribe controlled substances in the State of Maryland; and
(b) Either
registered or exempted from registration pursuant to 21 CFR §1301.22(c) and 21
CFR §1301.23.
(2) A prescription
issued by an authorized provider may be communicated to a pharmacist by an
employee or agent of the authorized provider.
B. Purpose of
Issue of Prescription.
(1) A controlled
substance prescription shall only be issued for a legitimate medical purpose by
a provider.
(2) A controlled
substance prescription shall be issued by a provider in the usual course of the
individual provider’s professional practice.
(3) The
responsibility for the proper prescribing and dispensing of controlled
substances is upon the prescribing provider.
(4) A pharmacist
has a corresponding responsibility to ensure, prior to dispensing, that a
controlled substance prescription is issued for a legitimate medical purpose.
(5) An order
purporting to be a prescription issued not in the usual course of professional
treatment or in legitimate and authorized research is not a prescription within
the meaning and intent of the Maryland Controlled Dangerous Substances Act,
Criminal Law Article, §§5-501—5-505, Annotated Code of Maryland.
(6) A prescription
may not be issued in order for an individual provider to obtain controlled
substances for supplying the individual provider for the purpose of general
dispensing to patients.
C. A prescription
for a Schedule II-V controlled substance shall be dispensed within 120 days
from the date of issue.
D. Unless the
State has stricter requirements, if a provider has complied with 21 CFR §1306,
the provider is deemed to have complied with this chapter.
.13 Partial Fill of a Prescription.
A partial fill of
a C-II prescription is permitted if:
A. The pharmacist
is unable to fill the full quantity of the prescription, pursuant to 21 CFR
§1306.13(a);
B. The
prescription is issued for a patient in a long-term care facility or for a
patient with a medical diagnosis documenting a terminal illness pursuant to 21
CFR 1306.13(b) or (c);
C. Requested by
the patient or the practitioner that wrote the prescription, pursuant to 21 CFR
§1306.13(b); or
D. The total
quantity dispensed in all partial fillings does not exceed the total quantity
prescribed.
.14 Refilling of Prescriptions.
A. A prescription
for a controlled substance listed in Schedule II may not be refilled.
B. A prescription
for a controlled substance listed in Schedule III, IV, or V:
(1) May not be
refilled more than 6 months after the date on which the prescription was
issued;
(2) May not be
refilled more than five times; and
(3) Shall require
a new and separate prescription to be issued if additional quantities are
deemed necessary by the authorized prescribing provider.
C. The prescribing
provider may authorize additional refills of Schedule III, IV, or V substances
on the original prescription through an oral refill authorization transmitted
to the pharmacist if the following conditions are met:
(1) The total
quantity authorized, including the amount of the original prescription, does
not exceed five refills or extend beyond 6 months from the date of issue of the
original prescription;
(2) The pharmacist
obtaining the oral authorization records on the reverse of the original paper
prescription or annotates the electronic prescription record with the date,
quantity of refill, and number of additional refills authorized, and initials
the paper prescription or annotates the electronic prescription record showing
who received the authorization from the prescribing practitioner who issued the
original prescription;
(3) The quantity
of each additional refill authorized is equal to or less than the quantity
authorized for the initial filling of the original prescription; and
(4) The
prescribing practitioner executes a new and separate prescription for any
additional quantities beyond the five-refill, 6-month limitation.
D. Required
Documentation.
(1) Each refilling
of a prescription shall be documented on the:
(a) Back of the
prescription;
(b) With
Department approval, on another appropriate uniformly maintained, readily
retrievable record, such as medication records; or
(c) electronic
prescription record.
(2) The
documentation shall indicate the:
(a) Number of the
prescription;
(b) Name of the
controlled substance;
(c) Dosage form of
the controlled substance;
(d) Date of each
refilling;
(e) Quantity
dispensed;
(f) Identity or
initials of the dispensing pharmacist in each refilling;
(g) Total number
of refills for that prescription;
(h) Pharmacist’s
initials; and
(i) Date by the
pharmacist as of the date of dispensing.
(3) Each refilling
of a prescription shall:
(a) State the
amount dispensed; or
(b) If the amount
dispensed is not specifically stated, be deemed to have been dispensed for the
full face amount of the prescription.
.15 Physical Security Controls for Registrants.
A registrant shall
comply with the provisions of 21 CFR §§1301.71—1301.77 as amended.
.16 Controlled Substance Disposal.
A. Any registrant
in possession of legally obtained controlled substances and desiring or
required to dispose of any of these substances may ask the Office of Controlled
Substances Administration of the Maryland Department of Health for authority
and instructions to dispose of this substance. This does not eliminate the
requirement on registrants under federal regulations to report destruction of
controlled dangerous substances.
B. Disposal of
Medical Waste.
(1) A registrant
in possession of legally obtained controlled substances deemed medical waste
shall render the controlled substances irretrievable by disposing of:
(a) Liquid waste
in an approved containment unit; or
(b) Non-liquid in
a receptacle.
(2) Two licensed
personnel shall keep Waste Destruction Logbooks and maintain the record for 2
years. The Destruction Log shall be readily available upon inspection.
C. Disposal for
Registrants Required to Register with the DEA.
(1) The Office of
Controlled Substances Administration shall authorize and instruct the
registrant to dispose of the controlled substance in one of the following
manners, provided complete records of the disposition are maintained by the
registrant:
(a) By transfer to
a person registered under the Act and authorized to possess the substance;
(b) By destruction
in the presence of an agent of the Office of Controlled Substances
Administration or another authorized person;
(c) By forwarding
to the District Office of the Drug Enforcement Administration pursuant to the
procedures of that agency;
(d) By returning
unused controlled substances dispensed by registered hospital pharmacies for
administration to in-patients to the pharmacy for appropriate disposition; or
(e) By other means
that the Secretary may determine to assure that the substance does not become
available to unauthorized persons.
(2) A registrant
that has complied with 21 CFR §1304 is deemed to have complied with this
section.
(3) If a
registrant is required regularly to dispose of controlled substances, the
Special Agent in Charge of the Federal Drug Enforcement Administration may
authorize the registrant to dispose of these substances, in accordance with 21
CFR §1307.
D. Disposal for
Registrants Not required to Register with the DEA. Any registrant who is
registered to administer only and is in possession of legally obtained
controlled substances shall:
(1) List the
controlled substance or substances which the registrant desires to dispose of
on a drug destruction form provided by the Department;
(2) Have two
members of the professional staff, limited to an administrator, nurse, or
pharmacist, destroy controlled drugs on premises;
(3) Dispose per
instructions given in §B of this regulation.
(4) Forward a copy
of the completed drug destruction form to the Office of Controlled Substances
Administration within 10 days of destruction; and
(5) Keep a copy of
the drug disposal form on location for a period of 2 years.
E. Abandoned
controlled substances may be:
(1) Destroyed in
the presence of an agent of the Office of Controlled Substances Administration
or another authorized person or persons;
(2) Disposed of by
other means that the Secretary may determine to assure that the substance does
not become available to unauthorized persons; or
(3) Impounded
under Health-General Article, §21-1113, Annotated Code of Maryland.
F. Aforementioned
procedures do not preclude release of medication to discharged patients if
ordered in writing by the attending physician.
.17 Transfer of Controlled Substances.
A. When closing
business activities, a registrant shall:
(1) Dispose of all
controlled dangerous substances in accordance with Regulation .16 of this
chapter; and
(2) Return the
controlled substances registration certificate to the Office of Controlled
Substances Administration inspector at the time of the closing inspection.
B. Change of
Ownership.
(1) A Change of
Ownership requires the registrant to notify the Department in writing,
Attention to the Office of Controlled Substances Administration.
(2) The written
notification shall be submitted:
(a) In person;
(b) By registered
mail; or
(c) By certified
mail with a return receipt request.
(3) The written
notification must be received 14 days before the transfer occurs.
(4) The Secretary
has the authority to waive the 14-day time limitation in individual instances.
(5) The written
notification shall contain the following information:
(a) The name;
(b) The address;
(c) The
registration number; and
(d) The authorized
business activity of the:
(i)
Registrant-transferor discontinuing the business; and
(ii)
Registrant-transferee acquiring the business;
(e) Whether the
business activities will be:
(i) Continued at
the location registered by the person discontinuing business; or
(ii) Moved to
another location and, if so, the address of the new location; and
(f) The date on
which the transfer of controlled substances will occur.
(6) Except for
transfer denials by the Secretary, the registrant-transferor may distribute,
without being registered to distribute, controlled substances in the
registrant-transferor’s possession to the registrant-transferee in accordance
with the following procedures, on the date of transfer of the controlled
substances:
(a) A complete
inventory of all controlled substances being transferred shall be taken and
serve as the final inventory of the registrant-transferor and the initial
inventory of the registrant-transferee;
(b) A copy of the
inventory shall be included in the records of both the transferor and
transferee;
(c) A copy of the
inventory shall be filed with the Department if requested by the Secretary;
(d) Transfers of
Schedule I or II controlled substances shall require the use of order forms in
accordance with 21 CFR §1305;
(e) All
transferor-controlled substance records shall be transferred to the transferee;
and
(f) Pursuant to 21
CFR §1304, transferors are required to make reports, which shall be marked as
“Final”.
.18 Administrative Inspections and Warrants.
A. Administrative
Inspections.
(1) Procedures
regarding administrative inspections and warrants pursuant to Criminal Law
Article, §§5-305, 5-804, and 5-805, Annotated Code of Maryland, are governed
generally by those sections of the Annotated Code and specifically by this
regulation.
(2) Authority to
make inspections is authorized in accordance with Criminal Law Article, §§5-804
and 5-805, Annotated Code of Maryland, to enter controlled premises and conduct
administrative inspections for the purpose of the following:
(a) Documents
required to be kept or made pursuant to 21 CFR §1304, under the Act, or
regulations promulgated under the Act may be:
(i) Inspected;
(ii) Copied;
(iii) Verified for
correctness; and
(iv) Inventoried;
(b) Collecting
samples of controlled substances or precursors (if samples are collected during
an inspection, the inspector shall issue a receipt for these samples to the
owner, operator, or agent in charge of the premises);
(c) Review of
records and information on the distribution of controlled substances by the
authorized provider;
(d) Reviewing the
authorized providers processes;
(e) Reviewing the
authorized providers controls; and
(f) Inspecting the
authorized providers facility operations;
(3) Entry.
(a) An inspection
shall be carried out by an inspector.
(b) An inspector
shall have the right to enter the premises and conduct inspections at
reasonable times and in a reasonable manner, upon the following conditions:
(i) Stating the
inspector’s purpose; and
(ii) Presenting to
the owner, operator, or agent in charge of the premises to be inspected,
appropriate credentials and written notice of the inspector’s inspection
authority under Criminal Law Article, §5-305, 5-804, or 5-805, Annotated Code
of Maryland.
(4) Consent to
Inspection of Financial Data, Sales Data Other than Shipment Data, or Pricing
Data.
(a) If inspection
is desired of financing, sales, or pricing data, written consent shall first be
obtained from the owner, operator, or agent in charge of the premises to be
inspected.
(b) This written
consent shall contain the following information:
(i) That the
owner, operator, or agent in charge has the right to refuse to consent to an
inspection of these items;
(ii) That anything
of an incriminating nature which may be found may be seized and used against
the owner, operator, or agent in charge in a criminal prosecution;
(iii) That the
owner, operator, or agent in charge has been presented with a notice of
inspection;
(iv) That the
consent is given by the owner, operator, or agent in charge voluntarily and
without threats of any kind; and
(v) That the
owner, operator, or agent in charge may withdraw the owner’s, operator’s, or
agent in charge’s consent at any time during the course of inspection.
(c) The written
consent shall be produced in duplicate and be distributed as follows:
(i) The original
will be retained by the inspector; and
(ii) The duplicate
will be given to the authorized provider inspected.
B. Administrative
Warrants.
(1) Probable
Cause. If the Department is satisfied that administrative probable cause
exists, the Department shall issue an administrative warrant.
(2) Execution of
Warrants. An administrative inspection warrant shall be executed as required by
Criminal Law Article, §5-804(d), Annotated Code of Maryland.
(3) Refusal to
Allow Inspection with an Administrative Warrant. If an authorized provider or
any person subject to the Act refuses to permit execution of an administrative
warrant, or impedes the inspector in the execution of that warrant, the
authorized provider or person shall be advised that this refusal or action
constitutes a violation of Criminal Law Article, §5-902(a)(3), Annotated Code
of Maryland.
.19 Hearings, Enforcement, and Disciplinary Sanctions.
A. Authority for
Enforcement Proceeding.
(1) A hearing may
be ordered or granted by the Secretary, at the Secretary’s discretion, to
permit any person against whom criminal or civil action is contemplated under
the Controlled Dangerous Substances Act an opportunity to present the
individual’s views and the individual’s proposals for bringing the individual’s
alleged violations into compliance with the law. The hearing will also permit
the individual to show cause why prosecution should not be instituted, or to
present the individual’s views on the contemplated proceeding.
(2) The rules of
procedure for hearings before the Secretary of the Maryland Department of
Health shall be governed by COMAR 10.01.03 and State Government Article,
§§10-201—10-226, Annotated Code of Maryland.
(3) Administrative
Hearings. Procedures for administrative hearings shall be governed by the rules
set forth in Criminal Law Article, §5-308, if applicable, Health-General
Article, §2-207, and State Government Article, §§10-201—10-226, Annotated Code
of Maryland, and COMAR 10.01.03.
B. After a hearing
pursuant to Criminal Law Article, §5-308, if applicable, and State Government
Article, §§10-201—10-226, Annotated Code of Maryland, and COMAR 10.01.03, the
Secretary may impose a sanction or penalty against any registrant that possesses
a Maryland-issued controlled substances registration pursuant to Criminal Law
Article, Title V, Annotated Code of Maryland, instead of or in addition to:
(1) The denial of
an application;
(2) The refusal to
renew a registration;
(3) Suspending a
registration;
(4) Revoking a
registration;
(5) Placing restrictions on a registration pursuant to Criminal Law
Article, §5-307, Annotated Code of Maryland; or
(6) Issuing a
nonpublic advisory letter.
C. Imposition of
Monetary Penalty.
(1) After a
hearing pursuant to Criminal Law Article, §5-308, Annotated Code of Maryland,
the Department may impose a monetary penalty against a controlled substance
registration holder pursuant to Criminal Law Article, §5-908, Annotated Code of
Maryland.
(2) The monetary
penalty may be imposed instead of or in addition to the:
(a) Suspension of
the controlled substances registration;
(b) Revocation of
the controlled substances registration; or
(c) Restrictions
placed on the controlled substances registration.
(3) The monetary
penalty shall be paid within 30 calendar days of the order, unless the order
specifies otherwise.
(4) Until the
imposed fine is paid, the registration may not be:
(a) Restored;
(b) Reinstated; or
(c) Renewed.
(5) In its
discretion, the Department may refer all cases of delinquent payment to the
Central Collection Unit of the Department of Budget and Management for
collection.
D. Notwithstanding
the guidelines set forth in this chapter, in order to resolve a pending
disciplinary action, the Department and the registrant may agree to a surrender
of a registration, or a consent order with terms, sanction, and penalties
agreed to by the Department and the registrant.
E. Nothing in this
chapter prohibits the Department from staying any period of non-active
suspension.
.20 Additional Controlled Substances in Maryland.
A. The following
substance, also known as a “bath salt”, is added to the Maryland Controlled
Dangerous Substances Act, Schedule I of the Criminal Law Article, §5-402,
Annotated Code of Maryland: F. 4-Methoxymethcathinone (Methedrone, bk-PMMA,
PMMC).
B. The following
substances are added to the Maryland Controlled Dangerous Substances Act,
Schedule III of the Criminal Law Article, §5-404, Annotated Code of Maryland:
(1) Butalbital,
Acetaminophen, caffeine C-III Fioricet®; and
(2) Opium < or
= 100mg per 100Gm or 100ml or not more than 5mg per dosage unit C-III.
LAURA HERRERA SCOTT
Secretary of Health
Special Documents
DEPARTMENT OF THE ENVIRONMENT
SUSQUEHANNA RIVER BASIN
COMMISSION
Commission
Meeting
AGENCY: Susquehanna
River Basin Commission.
ACTION: Notice.
SUMMARY: The
Susquehanna River Basin Commission will conduct its regular business meeting on
March 14, 2024 in Harrisburg, Pennsylvania.
Details concerning the matters to be addressed at the business meeting
are contained in the Supplementary Information section of this notice. Also the
Commission published a document in the Federal Register on January 10, 2024,
concerning its public hearing on February 1, 2024, in Harrisburg, Pennsylvania.
DATES: The meeting
will be held on Thursday, March 14, 2024, at 9 a.m.
ADDRESSES: This
public meeting will be conducted in person and digitally from the Susquehanna
River Basin Commission,4423 N. Front Street, Harrisburg, Pennsylvania.
FOR FURTHER INFORMATION CONTACT: Jason E. Oyler, General Counsel and Secretary
to the Commission, telephone: 717-238-0423; fax: 717-238-2436.
SUPPLEMENTARY INFORMATION:
The business meeting will include actions or presentations on the
following items: (1) approval of contracts, grants and agreements;
(2) request to ratify General Permit GP-03, Cooperative Fish Nursery
(3) a motion to release a proposed rulemaking for public comment; and
(4) actions on 25 regulatory program projects.
This agenda is complete at the
time of issuance, but other items may be added, and some stricken without
further notice. The listing of an item on the agenda does not necessarily mean
that the Commission will take final action on it at this meeting. When the
Commission does take final action, notice of these actions will be published in
the Federal Register after the meeting. Any actions specific to projects will
also be provided in writing directly to project sponsors.
The meeting will be conducted both
in person at the Susquehanna River Basin Commission, 4423 N. Front Street,
Harrisburg, Pennsylvania and digitally.
The public is invited to attend the Commission’s business meeting. You
can access the Business Meeting remotely via Zoom: https://us02web.zoom.us/j/89292000071?pwd=S1E2Qi9QNHUyTkhjY3ZoRUJJeXpqUT09
Meeting ID 892 9200 0071; Passcode: SRBC4423! or via telephone: 305-224-1968 or 309-205-3325; Meeting ID 892
9200 0071.
Written comments pertaining to
items on the agenda at the business meeting may be mailed to the Susquehanna
River Basin Commission, 4423 North Front Street, Harrisburg, Pennsylvania
17110-1788, or submitted electronically at the link Business
Meeting Comments. Such comments are due to the Commission on or
before March 11, 2024. Comments will not be accepted at the business meeting
noticed herein.
AUTHORITY: Pub. L.
91-575, 84 Stat. 1509 et seq., 18 CFR Parts 806, 807, and 808.
DATED: February 2, 2024.
JASON E. OYLER
General Counsel and Secretary to the Commission
[24-04-04]
WATER AND SCIENCE
ADMINISTRATION
Water
Quality Certification 23-WQC-0030
Pulaski Highway
968 Postal Road
Allentown, PA 18109
Add’l. Info: Pursuant to COMAR 26.08.02.10F(3)(c),
The Maryland Department of the Environment is providing notice of its issuance
of a Water Quality Certification 23-WQC-0030.
Location: Pulaski Highway (US Route 40 east), which
is north of Old Philadelphia Road east (MD Route 7, Bacon Hill area), Elkton,
in Cecil County.
The purpose of the project is to construct roads, paved
truck parking lots, culverts, outfalls, end walls, and retaining walls for a
407,500.000 square foot warehouse distribution facility, Jackson Property.
1. Permanent impact to 27,955
square feet of forested nontidal wetland, 10,398 square feet of forested
(isolated) nontidal wetland, 29,201 square feet of emergent nontidal wetland,
18,118 square feet of emergent (isolated) nontidal wetland, 148,379 square feet
of 25-foot nontidal wetland buffer, and 154 linear feet (542 square feet) of an
intermittent Use I stream (Mill Creek). Temporary impact to 2,256 square feet
of forested nontidal wetland, 32 square feet of emergent nontidal wetland, 15
square feet of emergent (isolated) nontidal wetland, and 5,497 square feet of
the 25-foot nontidal wetland buffer to Mill Creek (Use I).
The WQC and its attachments may be viewed at the following
link: https://mde.maryland.gov/programs/Water/WetlandsandWaterways/Pages/WQC.aspx
Appeal of Final Decision. This Water Quality Certification
is a final agency decision. Any person aggrieved by the Department’s decision
to issue this WQC may appeal such decision in accordance with COMAR
26.08.02.10F(4). A request for appeal shall be filed with the Department within
30 days of publication of the final decision and specify in writing the reason
why the final decision should be reconsidered. A request for appeal shall be
submitted to: Secretary of the Environment, Maryland Department of the Environment,
1800 Washington Boulevard, Baltimore, MD 21230. Any request for an appeal does
not stay the effectiveness of this WQC.
Contact: Louis Parnes at louis.parnes@maryland.gov
or 410-537-3786.
[24-04-09]
General Notices
Notice
of ADA Compliance
The State of Maryland is committed to
ensuring that individuals with disabilities are able to fully participate in
public meetings. Anyone planning to
attend a meeting announced below who wishes to receive auxiliary aids,
services, or accommodations is invited to contact the agency representative at
least 48 hours in advance, at the telephone number listed in the notice or
through Maryland Relay.
STATE COLLECTION AGENCY LICENSING BOARD
Subject: Public Meeting
Date and Time: March 12, 2024, 2
— 3 p.m.; Thereafter, the public meetings will take place the second Tuesday of
every month, accessed via the Google Meet information below.
Add’l. Info: Google Meet joining info:
Video link:
https://meet.google.com/ahz-mgnk-jsu
Or dial: (US) +1
530-738-1353‬
PIN: 815 799 863#
If necessary, the
Board will convene in a closed session to seek the advice of counsel or review
confidential materials, pursuant to General Provisions Article, §3-305,
Annotated Code of Maryland.
Contact: Ayanna Daugherty
410-230-6019
[24-04-03]
MARYLAND DEPARTMENT OF HEALTH
Subject: Public Meeting
Date
and Time: May 2,
2024, 9 a.m. — 1 p.m.
Place: Virtual meeting — please see
details below.
Add’l.
Info: Please be
advised that the May 2, 2024, Pharmacy and Therapeutics (P&T) Committee public meeting will be
conducted virtually via a Webinar.
As soon as
available, classes of drugs to be reviewed, speaker registration guidelines,
and procedure to register to attend the virtual meeting will be posted on the
Maryland Pharmacy Program website at
https://health.maryland.gov/mmcp/pap/Pages/Public-MeetingAnnouncement-and-Procedures-for-Public-Testimony.aspx.
Submit questions to:
mdh.marylandpdlquestions@maryland.gov.
Contact: Deborah Washington 410-767-1455
[24-04-13]
MARYLAND DEPARTMENT OF HEALTH/HARM REDUCTION STANDING
ADVISORY COMMITTEE
Subject: Public Meeting
Date and Time: March 8, 2024, 10
a.m. —12 p.m.
Place: Via Zoom — please see details below.
Add’l. Info: Log In:
https://us06web.zoom.us/j/86782287446?pwd=Ly03TKaRZHcl4OfLDOSr7UBSqVqZZT.1
Meeting ID: 867 8228 7446
Passcode: 388339
Contact: Katy Edwards,
katylaurel.edwards@maryland.gov
[24-04-02]
BOARD OF PUBLIC ACCOUNTANCY
Subject: Public Meeting
Date and Time: March 5, 2024, 9
a.m.
Place: Via Google Meet and in person at Maryland Dept. of
Labor, 1100 N. Eutaw St., 5th Fl., Baltimore, MD
Add’l. Info: Video link:
meet.google.com/yai-nvov-tdm
Or join by phone:
(US)+1 314-666-1864
PIN: 334 472 909#
Contact: Christopher E. Dorsey, Exec. Dir., 410-230-6318
[24-04-11]
BOARD OF PUBLIC ACCOUNTANCY
Subject: Public Hearing
Date and Time: March 5, 2024,
10:30 a.m. and 12:30 p.m.
Place: Maryland Dept. of Labor, 1100 N. Eutaw St., 5th Fl., Baltimore,
MD
Add’l. Info: The Board of Public Accountancy has scheduled two
application (reinstatement) hearings to take place at its March meeting.
Contact: Christopher E. Dorsey, Exec. Dir., 410-230-6318
[24-04-12]
STATE ADVISORY COUNCIL ON QUALITY CARE AT THE END OF
LIFE
Subject: Public Meeting
Date and Time: March 4, 2024, 10
a.m. — 12 p.m.
Place: Via Google Meet — please see details below.
Add’l. Info: Video link:
meet.google.com/aiv-dneh-vko
Or join by phone:
(US) +1
551-236-4103
PIN: 113 236 819#
The public is
welcome to attend the videoconference.
Contact: Paul Ballard
410-767-6918
[24-04-06]
BOARD OF WATERWORKS AND WASTE SYSTEMS OPERATORS
Subject: Public Meeting
Date and Time: March 21, 2024, 10
a.m. — 12p.m.
Place: Online via Google Chat — please see the Board’s
webpage for meeting details at:
https://mde.maryland.gov/programs/Permits/EnvironmentalBoards/Pages/BWW.aspx
Add’l. Info: A portion of this meeting may be held in closed session
Contact: J. Martin Fuhr
410-537-3588
[24-04-01]



